Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis
- PMID: 19923323
- PMCID: PMC2808235
- DOI: 10.1091/mbc.e09-03-0193
Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis
Abstract
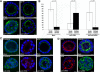
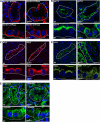
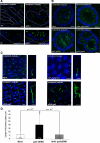
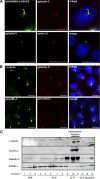
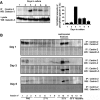
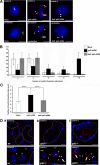
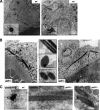
Galectin-3 is a beta-galactoside-binding protein widely expressed in all epithelia where it is involved in tissue homeostasis and cancer progression. We recently reported unique abnormalities in the identity of membrane domains in galectin-3 null mutant mice, suggesting that galectin-3 may participate in epithelial polarity program. We investigated the potential role of galectin-3 on early events in polarization of epithelial renal cells, using three-dimensional cultures of MDCK cells and also galectin-3 null mutant mouse kidneys. We show that depletion in galectin-3 systematically leads to severe perturbations of microtubular network associated with defects in membrane compartimentation, both in vitro and in vivo. Moreover, the absence of galectin-3 impinges on the morphology of the primary cilium, which is three times longer and unusually shaped. By immunological and biochemical approaches, we could demonstrate that endogenous galectin-3 is normally associated with basal bodies and centrosomes, where it closely interacts with core proteins, such as centrin-2. However, this association transiently occurs during the process of epithelial polarization. Interestingly, galectin-3-depleted cells contain numerous centrosome-like structures, demonstrating an unexpected function of this protein in the formation and/or stability of the centrosomes. Collectively, these data establish galectin-3 as a key determinant in epithelial morphogenesis via its effect on centrosome biology.
Figures
Similar articles
-
Galectin-3 associates with the primary cilium and modulates cyst growth in congenital polycystic kidney disease.Am J Pathol. 2006 Dec;169(6):1925-38. doi: 10.2353/ajpath.2006.060245. Am J Pathol. 2006. PMID: 17148658 Free PMC article.
-
Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo.J Cell Sci. 2008 Feb 15;121(Pt 4):458-65. doi: 10.1242/jcs.020800. Epub 2008 Jan 22. J Cell Sci. 2008. PMID: 18211959
-
Transcriptional program of ciliated epithelial cells reveals new cilium and centrosome components and links to human disease.PLoS One. 2012;7(12):e52166. doi: 10.1371/journal.pone.0052166. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300604 Free PMC article.
-
Recycling of galectin-3 in epithelial cells.Eur J Cell Biol. 2015 Jul-Sep;94(7-9):309-15. doi: 10.1016/j.ejcb.2015.05.004. Epub 2015 Jun 1. Eur J Cell Biol. 2015. PMID: 26059399 Review.
-
The Centrosome as a Geometry Organizer.Results Probl Cell Differ. 2019;67:253-276. doi: 10.1007/978-3-030-23173-6_11. Results Probl Cell Differ. 2019. PMID: 31435799 Review.
Cited by
-
The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation.Front Cell Dev Biol. 2020 Apr 15;8:253. doi: 10.3389/fcell.2020.00253. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32351961 Free PMC article. Review.
-
LGALS3BP regulates centriole biogenesis and centrosome hypertrophy in cancer cells.Nat Commun. 2013;4:1531. doi: 10.1038/ncomms2517. Nat Commun. 2013. PMID: 23443559
-
Structure-function analysis of the EF-hand protein centrin-2 for its intracellular localization and nucleotide excision repair.Nucleic Acids Res. 2013 Aug;41(14):6917-29. doi: 10.1093/nar/gkt434. Epub 2013 May 28. Nucleic Acids Res. 2013. PMID: 23716636 Free PMC article.
-
Galectin-7 modulates the length of the primary cilia and wound repair in polarized kidney epithelial cells.Am J Physiol Renal Physiol. 2011 Sep;301(3):F622-33. doi: 10.1152/ajprenal.00134.2011. Epub 2011 Jun 15. Am J Physiol Renal Physiol. 2011. PMID: 21677144 Free PMC article.
-
Intracellular galectin interactions in health and disease.Semin Immunopathol. 2024 Jul 11;46(1-2):4. doi: 10.1007/s00281-024-01010-z. Semin Immunopathol. 2024. PMID: 38990375 Free PMC article. Review.
References
-
- Assemat E., Bazellieres E., Pallesi-Pocachard E., Le Bivic A., Massey-Harroche D. Polarity complex proteins. Biochim. Biophys. Acta. 2008;1778:614–630. - PubMed
-
- Azimzadeh J., Bornens M. Structure and duplication of the centrosome. J. Cell Sci. 2007;120:2139–2142. - PubMed
-
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

