A FAM21-containing WASH complex regulates retromer-dependent sorting
- PMID: 19922874
- PMCID: PMC2803077
- DOI: 10.1016/j.devcel.2009.09.009
A FAM21-containing WASH complex regulates retromer-dependent sorting
Abstract
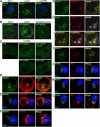
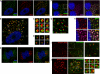
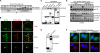
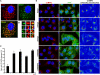
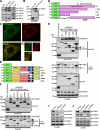
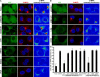
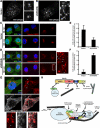
The Arp2/3 complex regulates endocytosis, sorting, and trafficking, yet the Arp2/3-stimulating factors orchestrating these distinct events remain ill defined. WASH (Wiskott-Aldrich Syndrome Protein and SCAR Homolog) is an Arp2/3 activator with unknown function that was duplicated during primate evolution. We demonstrate that WASH associates with tubulin and localizes to early endosomal subdomains, which are enriched in Arp2/3, F-actin, and retromer components. Although WASH localized with activated receptors, it was not essential for endocytosis. However, WASH did regulate retromer-mediated retrograde CI-MPR trafficking, which required its association with endosomes, Arp2/3-directed F-actin regulation, and tubulin interaction. Moreover, WASH exists in a multiprotein complex containing FAM21, which links WASH to endosomes and is required for WASH-dependent retromer-mediated sorting. Significantly, without WASH, retromer tubulation was exaggerated, supporting a model wherein WASH links retromer-mediated cargo containing tubules to microtubules for Golgi-directed trafficking and generates F-actin-driven force for tubule scission.
Figures
Comment in
-
Sorting out endosomes in the WASH.Dev Cell. 2009 Nov;17(5):583-4. doi: 10.1016/j.devcel.2009.10.019. Dev Cell. 2009. PMID: 19922862
Similar articles
-
Endosomal recruitment of the WASH complex: active sequences and mutations impairing interaction with the retromer.Biol Cell. 2013 May;105(5):191-207. doi: 10.1111/boc.201200038. Epub 2013 Mar 7. Biol Cell. 2013. PMID: 23331060
-
Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer.Mol Biol Cell. 2012 Jun;23(12):2352-61. doi: 10.1091/mbc.E11-12-1059. Epub 2012 Apr 18. Mol Biol Cell. 2012. PMID: 22513087 Free PMC article.
-
Recruitment of the endosomal WASH complex is mediated by the extended 'tail' of Fam21 binding to the retromer protein Vps35.Biochem J. 2012 Feb 15;442(1):209-20. doi: 10.1042/BJ20111761. Biochem J. 2012. PMID: 22070227
-
Actin-dependent endosomal receptor recycling.Curr Opin Cell Biol. 2019 Feb;56:22-33. doi: 10.1016/j.ceb.2018.08.006. Epub 2018 Sep 15. Curr Opin Cell Biol. 2019. PMID: 30227382 Review.
-
Retromer-mediated endosomal protein sorting: all WASHed up!Trends Cell Biol. 2013 Nov;23(11):522-8. doi: 10.1016/j.tcb.2013.04.010. Epub 2013 May 28. Trends Cell Biol. 2013. PMID: 23721880 Free PMC article. Review.
Cited by
-
ER as master regulator of membrane trafficking and organelle function.J Cell Biol. 2022 Oct 3;221(10):e202205135. doi: 10.1083/jcb.202205135. Epub 2022 Sep 15. J Cell Biol. 2022. PMID: 36108241 Free PMC article. Review.
-
Assembly and Activity of the WASH Molecular Machine: Distinctive Features at the Crossroads of the Actin and Microtubule Cytoskeletons.Front Cell Dev Biol. 2021 Apr 1;9:658865. doi: 10.3389/fcell.2021.658865. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869225 Free PMC article. Review.
-
VPS35 pathogenic mutations confer no dominant toxicity but partial loss of function in Drosophila and genetically interact with parkin.Hum Mol Genet. 2015 Nov 1;24(21):6106-17. doi: 10.1093/hmg/ddv322. Epub 2015 Aug 6. Hum Mol Genet. 2015. PMID: 26251041 Free PMC article.
-
A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration.Neurogenetics. 2015 Jul;16(3):215-221. doi: 10.1007/s10048-015-0446-0. Epub 2015 Apr 17. Neurogenetics. 2015. PMID: 25894286 Free PMC article.
-
Retromer Controls Planar Polarity Protein Levels and Asymmetric Localization at Intercellular Junctions.Curr Biol. 2019 Feb 4;29(3):484-491.e6. doi: 10.1016/j.cub.2018.12.027. Epub 2019 Jan 17. Curr Biol. 2019. PMID: 30661800 Free PMC article.
References
-
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–1800. - PubMed
-
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

