Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ
- PMID: 19920187
- PMCID: PMC2789178
- DOI: 10.1158/0008-5472.CAN-09-1043
Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ
Abstract
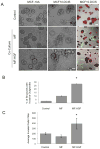
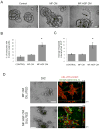
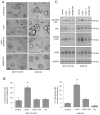
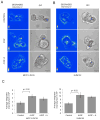
Stromal-derived hepatocyte growth factor (HGF) acting through its specific proto-oncogene receptor c-Met has been suggested to play a paracrine role in the regulation of tumor cell migration and invasion. The transition from preinvasive ductal carcinoma in situ (DCIS) to invasive breast carcinoma is marked by infiltration of stromal fibroblasts and the loss of basement membrane. We hypothesized that HGF produced by the infiltrating fibroblasts may alter proteolytic pathways in DCIS cells, and, to study this hypothesis, established three-dimensional reconstituted basement membrane overlay cocultures with two human DCIS cell lines, MCF10.DCIS and SUM102. Both cell lines formed large dysplastic structures in three-dimensional cultures that resembled DCIS in vivo and occasionally developed invasive outgrowths. In coculture with HGF-secreting mammary fibroblasts, the percentage of DCIS structures with invasive outgrowths was increased. Activation of c-Met with conditioned medium from HGF-secreting fibroblasts or with recombinant HGF increased the percentage of DCIS structures with invasive outgrowths, their degradation of collagen IV, and their secretion of urokinase-type plasminogen activator and its receptor. In agreement with the in vitro findings, coinjection with HGF-secreting fibroblasts increased invasiveness of MCF10.DCIS xenografts in severe combined immunodeficient mice. Our study shows that paracrine HGF/c-Met signaling between fibroblasts and preinvasive DCIS cells enhances the transition to invasive carcinomas and suggests that three-dimensional cocultures are appropriate models for testing therapeutics that target tumor microenvironment-enhanced invasiveness.
Conflict of interest statement
Figures


Similar articles
-
MAME models for 4D live-cell imaging of tumor: microenvironment interactions that impact malignant progression.J Vis Exp. 2012 Feb 17;(60):3661. doi: 10.3791/3661. J Vis Exp. 2012. PMID: 22371028 Free PMC article.
-
Stromal cells associated with early invasive foci in human mammary ductal carcinoma in situ coexpress urokinase and urokinase receptor.Int J Cancer. 2007 May 15;120(10):2086-95. doi: 10.1002/ijc.22340. Int J Cancer. 2007. PMID: 17290405
-
Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancers through paracrine activation of Met.Breast Cancer Res. 2012 Jul 12;14(4):R104. doi: 10.1186/bcr3224. Breast Cancer Res. 2012. PMID: 22788954 Free PMC article.
-
Biological mechanisms in breast cancer invasiveness: relevance to preventive interventions.Eur J Cancer Prev. 2000 Apr;9(2):73-9. doi: 10.1097/00008469-200004000-00002. Eur J Cancer Prev. 2000. PMID: 10830573 Review.
-
Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions.Int J Cancer. 2006 Aug 1;119(3):477-83. doi: 10.1002/ijc.21808. Int J Cancer. 2006. PMID: 16453287 Review.
Cited by
-
Insidious changes in stromal matrix fuel cancer progression.Mol Cancer Res. 2014 Mar;12(3):297-312. doi: 10.1158/1541-7786.MCR-13-0535. Epub 2014 Jan 22. Mol Cancer Res. 2014. PMID: 24452359 Free PMC article. Review.
-
Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: possible role of the TGF-β1/TGF-βR1 pathway.BMC Cancer. 2014 Jul 9;14:499. doi: 10.1186/1471-2407-14-499. BMC Cancer. 2014. PMID: 25011545 Free PMC article.
-
Tumor budding in colorectal carcinoma assessed by cytokeratin immunostaining and budding areas: possible involvement of c-Met.Cancer Sci. 2014 Nov;105(11):1487-95. doi: 10.1111/cas.12530. Epub 2014 Oct 9. Cancer Sci. 2014. PMID: 25220207 Free PMC article.
-
MAME models for 4D live-cell imaging of tumor: microenvironment interactions that impact malignant progression.J Vis Exp. 2012 Feb 17;(60):3661. doi: 10.3791/3661. J Vis Exp. 2012. PMID: 22371028 Free PMC article.
-
Anti-cancer therapeutic strategies based on HGF/MET, EpCAM, and tumor-stromal cross talk.Cancer Cell Int. 2022 Aug 19;22(1):259. doi: 10.1186/s12935-022-02658-z. Cancer Cell Int. 2022. PMID: 35986321 Free PMC article. Review.
References
-
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. - PubMed
-
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. - PubMed
-
- Gao CF, Vande Woude GF. HGF/SF-Met signaling in tumor progression. Cell research. 2005;15:49–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

