The GIT-PIX complexes regulate the chemotactic response of rat basophilic leukaemia cells
- PMID: 19912111
- PMCID: PMC2825732
- DOI: 10.1042/BC20090074
The GIT-PIX complexes regulate the chemotactic response of rat basophilic leukaemia cells
Abstract
Background information: Cell motility entails the reorganization of the cytoskeleton and membrane trafficking for effective protrusion. The GIT-PIX protein complexes are involved in the regulation of cell motility and adhesion and in the endocytic traffic of members of the family of G-protein-coupled receptors. We have investigated the function of the endogenous GIT complexes in the regulation of cell motility stimulated by fMLP (formyl-Met-Leu-Phe) peptide, in a rat basophilic leukaemia RBL-2H3 cell line stably expressing an HA (haemagglutinin)-tagged receptor for the fMLP peptide.
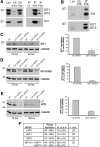
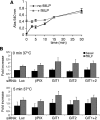
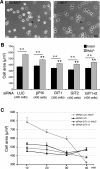
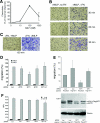
Results: Our analysis shows that RBL cells stably transfected with the chemoattractant receptor expressed both GIT1-PIX and GIT2-PIX endogenous complexes. We have used silencing of the different members of the complex by small interfering RNAs to study the effects on a number of events linked to agonist-induced cell migration. We found that cell adhesion was not affected by depletion of any of the proteins of the GIT complex, whereas agonist-enhanced cell spreading was inhibited. Analysis of agonist-stimulated haptotactic cell migration indicated a specific positive effect of GIT1 depletion on trans-well migration. The internalization of the formyl-peptide receptor was also inhibited by depletion of GIT1 and GIT2. The effects of the GIT complexes on trafficking of the receptors was confirmed by an antibody-enhanced agonist-induced internalization assay, showing that depletion of PIX, GIT1 or GIT2 protein caused decreased perinuclear accumulation of internalized receptors.
Conclusions: Our results show that endogenous GIT complexes are involved in the regulation of chemoattractant-induced cell motility and receptor trafficking, and support previous findings indicating an important function of the GIT complexes in the regulation of different G-protein-coupled receptors. Our results also indicate that endogenous GIT1 and GIT2 regulate distinct subsets of agonist-induced responses and suggest a possible functional link between the control of receptor trafficking and the regulation of cell motility by GIT proteins.
Figures
Similar articles
-
Expanding functions of GIT Arf GTPase-activating proteins, PIX Rho guanine nucleotide exchange factors and GIT-PIX complexes.J Cell Sci. 2016 May 15;129(10):1963-74. doi: 10.1242/jcs.179465. J Cell Sci. 2016. PMID: 27182061 Free PMC article. Review.
-
The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors.Cell Signal. 2004 Sep;16(9):1001-11. doi: 10.1016/j.cellsig.2004.02.002. Cell Signal. 2004. PMID: 15212761
-
Sorting nexin 27 protein regulates trafficking of a p21-activated kinase (PAK) interacting exchange factor (β-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction.J Biol Chem. 2011 Nov 11;286(45):39403-16. doi: 10.1074/jbc.M111.260802. Epub 2011 Sep 18. J Biol Chem. 2011. PMID: 21926430 Free PMC article.
-
β-PIX plays an important role in regulation of intestinal epithelial restitution by interacting with GIT1 and Rac1 after wounding.Am J Physiol Gastrointest Liver Physiol. 2018 Mar 1;314(3):G399-G407. doi: 10.1152/ajpgi.00296.2017. Epub 2017 Nov 30. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29191942 Free PMC article.
-
The multifunctional GIT family of proteins.J Cell Sci. 2006 Apr 15;119(Pt 8):1469-75. doi: 10.1242/jcs.02925. J Cell Sci. 2006. PMID: 16598076 Review.
Cited by
-
Biochemical and functional characterization of the interaction between liprin-α1 and GIT1: implications for the regulation of cell motility.PLoS One. 2011;6(6):e20757. doi: 10.1371/journal.pone.0020757. Epub 2011 Jun 13. PLoS One. 2011. PMID: 21695141 Free PMC article.
-
Expanding functions of GIT Arf GTPase-activating proteins, PIX Rho guanine nucleotide exchange factors and GIT-PIX complexes.J Cell Sci. 2016 May 15;129(10):1963-74. doi: 10.1242/jcs.179465. J Cell Sci. 2016. PMID: 27182061 Free PMC article. Review.
-
GIT2 deficiency attenuates concanavalin A-induced hepatitis in mice.FEBS Open Bio. 2015 Aug 11;5:688-704. doi: 10.1016/j.fob.2015.08.005. eCollection 2015. FEBS Open Bio. 2015. PMID: 26380813 Free PMC article.
-
Identification of a Protein Network Driving Neuritogenesis of MGE-Derived GABAergic Interneurons.Front Cell Neurosci. 2016 Dec 21;10:289. doi: 10.3389/fncel.2016.00289. eCollection 2016. Front Cell Neurosci. 2016. PMID: 28066185 Free PMC article.
-
Regulation of microtubule nucleation in mouse bone marrow-derived mast cells by ARF GTPase-activating protein GIT2.Front Immunol. 2024 Feb 2;15:1321321. doi: 10.3389/fimmu.2024.1321321. eCollection 2024. Front Immunol. 2024. PMID: 38370406 Free PMC article.
References
-
- Ali H., Sozzani S., Fisher I., Barr A.J., Richardson M., Haribabu B., Snyderman R. Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase Cbeta3 phosphorylation by protein kinase A. J. Biol. Chem. 1998;273:11012–11016. - PubMed
-
- Bagrodia S., Taylor S.J., Jordon K.A., Van Aelst L., Cerione R.A. A novel regulator of p21-activated kinases. J. Biol. Chem. 1998;273:23633–23636. - PubMed
-
- Botrugno O.A., Paris S., Za L., Gualdoni S., Cattaneo A., Bachi A., de Curtis I. Characterization of the endogenous GIT1-betaPIX complex, and identification of its association to membranes. Eur. J. Cell Biol. 2006;85:35–46. - PubMed
-
- Bretscher M.S., Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr. Biol. 1998;8:721–724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

