Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans
- PMID: 19900588
- PMCID: PMC3638869
- DOI: 10.1016/j.mito.2009.11.003
Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans
Abstract
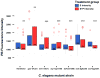
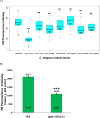
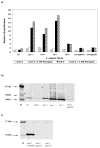
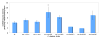
Mitochondrial dysfunction and associated oxidant stress have been linked with numerous complex diseases and aging largely by in vitro determination of mitochondria oxidant production and scavenging. We applied targeted in vivo fluorescence analyses of mitochondria-dense pharyngeal tissue in Caenorhabditis elegans to better understand relative mitochondrial effects, particularly on matrix oxidant burden, of respiratory chain complex, MnSOD, and insulin receptor mutants displaying variable longevity. The data demonstrate significantly elevated in vivo matrix oxidant burden in the short-lived complex I mutant, gas-1(fc21), which was associated with limited superoxide scavenging capacity despite robust MnSOD induction, as well as decreased mitochondria content and membrane potential. Significantly increased MnSOD activity was associated with in vivo matrix oxidant levels similar to wild-type in the long-lived respiratory chain complex III mutant, isp-1(qm150). Yet, despite greater superoxide scavenging capacity in the complex III mutant than in the significantly longer-lived insulin receptor mutant, daf-2(e1368), only the former showed modest oxidative stress sensitivity. Furthermore, increased longevity was seen in MnSOD knockout mutants (sod-2(ok1030) and sod-2(gk257)) that had decreased MnSOD scavenging capacity and increased in vivo matrix oxidant burden. Thus, factors beside oxidant stress must underlie RC mutant longevity in C. elegans. This work highlights the utility of the C. elegans model as a tractable means to non-invasively monitor multi-dimensional in vivo consequences of primary mitochondrial dysfunction.
Figures

Similar articles
-
Uncoupling of oxidative stress resistance and lifespan in long-lived isp-1 mitochondrial mutants in Caenorhabditis elegans.Free Radic Biol Med. 2017 Jul;108:362-373. doi: 10.1016/j.freeradbiomed.2017.04.004. Epub 2017 Apr 7. Free Radic Biol Med. 2017. PMID: 28392283 Free PMC article.
-
Pharmacologic targeting of sirtuin and PPAR signaling improves longevity and mitochondrial physiology in respiratory chain complex I mutant Caenorhabditis elegans.Mitochondrion. 2015 May;22:45-59. doi: 10.1016/j.mito.2015.02.005. Epub 2015 Mar 3. Mitochondrion. 2015. PMID: 25744875 Free PMC article.
-
A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans.PLoS Biol. 2010 Dec 7;8(12):e1000556. doi: 10.1371/journal.pbio.1000556. PLoS Biol. 2010. PMID: 21151885 Free PMC article.
-
Mitochondrial respiration and reactive oxygen species in C. elegans.Exp Gerontol. 2006 Oct;41(10):957-67. doi: 10.1016/j.exger.2006.06.056. Epub 2006 Aug 21. Exp Gerontol. 2006. PMID: 16919906 Review.
-
Effects of the mitochondrial respiratory chain on longevity in C. elegans.Exp Gerontol. 2014 Aug;56:245-55. doi: 10.1016/j.exger.2014.03.028. Epub 2014 Apr 5. Exp Gerontol. 2014. PMID: 24709342 Review.
Cited by
-
Inhibiting cytosolic translation and autophagy improves health in mitochondrial disease.Hum Mol Genet. 2015 Sep 1;24(17):4829-47. doi: 10.1093/hmg/ddv207. Epub 2015 Jun 3. Hum Mol Genet. 2015. PMID: 26041819 Free PMC article.
-
Effects of early life exposure to ultraviolet C radiation on mitochondrial DNA content, transcription, ATP production, and oxygen consumption in developing Caenorhabditis elegans.BMC Pharmacol Toxicol. 2013 Feb 4;14:9. doi: 10.1186/2050-6511-14-9. BMC Pharmacol Toxicol. 2013. PMID: 23374645 Free PMC article.
-
Structure and biological evaluation of Caenorhabditis elegans CISD-1/mitoNEET, a KLP-17 tail domain homologue, supports attenuation of paraquat-induced oxidative stress through a p38 MAPK-mediated antioxidant defense response.Adv Redox Res. 2022 Dec;6:100048. doi: 10.1016/j.arres.2022.100048. Epub 2022 Sep 23. Adv Redox Res. 2022. PMID: 36533211 Free PMC article.
-
Increased mitochondrial fusion allows the survival of older animals in diverse C. elegans longevity pathways.Nat Commun. 2017 Aug 3;8(1):182. doi: 10.1038/s41467-017-00274-4. Nat Commun. 2017. PMID: 28769038 Free PMC article.
-
Comparative Analysis of Experimental Methods to Quantify Animal Activity in Caenorhabditis elegans Models of Mitochondrial Disease.J Vis Exp. 2021 Apr 4;(170):10.3791/62244. doi: 10.3791/62244. J Vis Exp. 2021. PMID: 33871460 Free PMC article.
References
-
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275(938):299–325. - PubMed
-
- Altun ZF, Hall DH. Alimentary System: Pharynx. WormAtlas. 2008. http://www.wormatlas.org/hermaphrodite/pharynx/mainframe.htm.
-
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3(4):473–85. - PubMed
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

