Retinoblastoma family proteins have distinct functions in pulmonary epithelial cells in vivo critical for suppressing cell growth and tumorigenesis
- PMID: 19887614
- PMCID: PMC2778863
- DOI: 10.1158/0008-5472.CAN-09-1359
Retinoblastoma family proteins have distinct functions in pulmonary epithelial cells in vivo critical for suppressing cell growth and tumorigenesis
Abstract
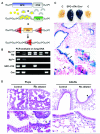
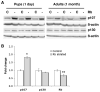
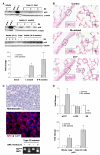
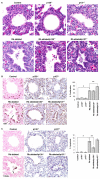
Lung cancer is the leading cause of cancer deaths, accounting for more deaths than breast, colon, and prostate cancer combined. The retinoblastoma (Rb)/p16 tumor suppressive pathway is deregulated in most cancers. Loss of p16 occurs more frequently than Rb loss, suggesting that p16 suppresses cancer by regulating Rb as well as the related proteins p107 and p130. However, direct evidence demonstrating that p130 or p107 cooperate with Rb to suppress epithelial cancers associated with p16 loss is currently lacking. Moreover, the roles of p130 and p107 in lung cancer are not clear. In the present studies, Rb ablation was targeted to the lung epithelium in wild-type, p107, or p130 null mice to determine unique and overlapping Rb family functions critical in tumor suppression. Rb ablation during development resulted in marked epithelial abnormalities despite p107 upregulation. In contrast, p130 and p107 were not required during development but had distinct functions in the Rb-deficient epithelium: p107 was required to suppress proliferation, whereas a novel proapoptotic function was identified for p130. Adult Rb-ablated lungs lacked the epithelial phenotype seen at birth and showed compensatory p107 upregulation and p16 induction in epithelial cell lineages that share phenotypic characteristics with human non-small cell lung cancers (NSCLC) that frequently show p16 loss. Importantly, Rb/p107-deficient, but not Rb/p130-deficient, lungs developed tumors resembling NSCLC. Taken together, these studies identify distinct Rb family functions critical in controlling epithelial cell growth, and provide direct evidence that p107 cooperates with Rb to protect against a common adult cancer.
Figures
Similar articles
-
The specific role of pRb in p16 (INK4A) -mediated arrest of normal and malignant human breast cells.Cell Cycle. 2012 Mar 1;11(5):1008-13. doi: 10.4161/cc.11.5.19492. Epub 2012 Mar 1. Cell Cycle. 2012. PMID: 22333593 Free PMC article.
-
Retinoblastoma/p107/p130 pocket proteins: protein dynamics and interactions with target gene promoters.J Biol Chem. 2009 Jul 17;284(29):19265-71. doi: 10.1074/jbc.M808740200. Epub 2009 Mar 11. J Biol Chem. 2009. PMID: 19279001 Free PMC article.
-
The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium.J Biol Chem. 2006 Jan 6;281(1):638-47. doi: 10.1074/jbc.M509053200. Epub 2005 Oct 28. J Biol Chem. 2006. PMID: 16258171
-
p107 and p130: versatile proteins with interesting pockets.Exp Cell Res. 2001 Mar 10;264(1):135-47. doi: 10.1006/excr.2000.5135. Exp Cell Res. 2001. PMID: 11237530 Review.
-
From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway.J Cell Biochem. 2007 Dec 15;102(6):1400-4. doi: 10.1002/jcb.21609. J Cell Biochem. 2007. PMID: 17979151 Review.
Cited by
-
Short somatic alterations at the site of copy number variation in breast cancer.Cancer Sci. 2021 Jan;112(1):444-453. doi: 10.1111/cas.14630. Epub 2020 Dec 11. Cancer Sci. 2021. PMID: 32860329 Free PMC article.
-
Regulation of lung endoderm progenitor cell behavior by miR302/367.Development. 2011 Apr;138(7):1235-45. doi: 10.1242/dev.061762. Epub 2011 Feb 24. Development. 2011. PMID: 21350014 Free PMC article.
-
TRP53 Mutants Drive Neuroendocrine Lung Cancer Through Loss-of-Function Mechanisms with Gain-of-Function Effects on Chemotherapy Response.Mol Cancer Ther. 2017 Dec;16(12):2913-2926. doi: 10.1158/1535-7163.MCT-17-0353. Epub 2017 Aug 28. Mol Cancer Ther. 2017. PMID: 28847987 Free PMC article.
-
Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity.Cancer Cell. 2021 Aug 9;39(8):1115-1134.e12. doi: 10.1016/j.ccell.2021.06.016. Epub 2021 Jul 21. Cancer Cell. 2021. PMID: 34270926 Free PMC article.
-
Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway.PLoS One. 2010 Apr 20;5(4):e10243. doi: 10.1371/journal.pone.0010243. PLoS One. 2010. PMID: 20421925 Free PMC article.
References
-
- Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. - PubMed
-
- Kleinerman RA, Tarone RE, Abramson DH, Seddon JM, Li FP, Tucker MA. Hereditary retinoblastoma and risk of lung cancer. J Natl Cancer Inst. 2000;92:2037–9. - PubMed
-
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. - PubMed
-
- Knudsen ES, Knudsen KE. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Exp Biol Med (Maywood) 2006;231:1271–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

