Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer
- PMID: 19887483
- PMCID: PMC3644858
- DOI: 10.1158/1078-0432.CCR-09-1722
Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer
Abstract
Purpose: Increasing evidence indicates that enhanced intratumoral androgen synthesis contributes to prostate cancer progression after androgen deprivation therapy. This phase II study was designed to assess responses to blocking multiple steps in androgen synthesis with inhibitors of CYP17A1 (ketoconazole) and type I and II 5alpha-reductases (dutasteride) in patients with castration-resistant prostate cancer (CRPC).
Experimental design: Fifty-seven men with CRPC were continued on gonadal suppression and treated with ketoconazole (400 mg thrice daily), hydrocortisone (30 mg/AM, 10 mg/PM), and dutasteride (0.5 mg/d).
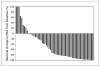
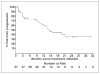
Results: Prostate-specific antigen response rate (> or =50% decline) was 56% (32 of 57; 95% confidence interval, 42.4-69.3%); the median duration of response was 20 months. In patients with measurable disease, 6 of 20 (30%) responded by the Response Evaluation Criteria in Solid Tumors. Median duration of treatment was 8 months; 9 patients remained on therapy with treatment durations censored at 18 to 32 months. Median time to progression was 14.5 months. Grade 3 toxicities occurred in 32% with only one reported grade 4 (thrombosis) toxicity. Dehydroepiandrosterone sulfate declined by 89%, androstenedione by 56%, and testosterone by 66%, and dihydrotestosterone declined to below detectable levels compared with baseline levels with testicular suppression alone. Median baseline levels and declines in dehydroepiandrosterone sulfate, androstenedione, testosterone, and dihydrotestosterone were not statistically different in the responders versus nonresponders, and hormone levels were not significantly increased from nadir levels at relapse.
Conclusion: The response proportion to ketoconazole, hydrocortisone, and dutasteride was at least comparable with previous studies of ketoconazole alone, whereas time to progression was substantially longer. Combination therapies targeting multiple steps in androgen synthesis warrant further investigation.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Targeted androgen pathway suppression in localized prostate cancer: a pilot study.J Clin Oncol. 2014 Jan 20;32(3):229-37. doi: 10.1200/JCO.2012.48.6431. Epub 2013 Dec 9. J Clin Oncol. 2014. PMID: 24323034 Free PMC article. Clinical Trial.
-
Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer.J Urol. 2002 Aug;168(2):542-5. J Urol. 2002. PMID: 12131305
-
Phase II study of Dutasteride for recurrent prostate cancer during androgen deprivation therapy.J Urol. 2009 Feb;181(2):621-6. doi: 10.1016/j.juro.2008.10.014. Epub 2008 Dec 16. J Urol. 2009. PMID: 19091347 Free PMC article. Clinical Trial.
-
The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer.J Urol. 2008 Apr;179(4):1235-42. doi: 10.1016/j.juro.2007.11.033. Epub 2008 Feb 20. J Urol. 2008. PMID: 18280514 Free PMC article. Review.
-
Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia.World J Urol. 2002 Apr;19(6):413-25. doi: 10.1007/s00345-002-0248-5. World J Urol. 2002. PMID: 12022710 Review.
Cited by
-
[Change of the LHRH analogue in progressive castration-refractory prostate cancer].Urologe A. 2012 Sep;51(9):1282-7. doi: 10.1007/s00120-012-2948-9. Urologe A. 2012. PMID: 22733398 Clinical Trial. German.
-
Contemporary experience with ketoconazole in patients with metastatic castration-resistant prostate cancer: clinical factors associated with PSA response and disease progression.Prostate. 2012 Mar;72(4):461-7. doi: 10.1002/pros.21447. Epub 2011 Jun 17. Prostate. 2012. PMID: 21688281 Free PMC article.
-
Evolving standards in the treatment of docetaxel-refractory castration-resistant prostate cancer.Prostate Cancer Prostatic Dis. 2011 Sep;14(3):192-205. doi: 10.1038/pcan.2011.23. Epub 2011 May 17. Prostate Cancer Prostatic Dis. 2011. PMID: 21577234 Free PMC article. Review.
-
NFX1-123: A potential therapeutic target in cervical cancer.J Med Virol. 2023 Jun;95(6):e28856. doi: 10.1002/jmv.28856. J Med Virol. 2023. PMID: 37288708 Free PMC article.
-
Repurposing antifungal drugs for cancer therapy.J Adv Res. 2023 Jun;48:259-273. doi: 10.1016/j.jare.2022.08.018. Epub 2022 Sep 5. J Adv Res. 2023. PMID: 36067975 Free PMC article. Review.
References
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. - PubMed
-
- Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. - PubMed
-
- Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–6. - PubMed
-
- Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical