Complex regulation of the transactivation function of hypoxia-inducible factor-1 alpha by direct interaction with two distinct domains of the CREB-binding protein/p300
- PMID: 19880525
- PMCID: PMC2807317
- DOI: 10.1074/jbc.M109.021824
Complex regulation of the transactivation function of hypoxia-inducible factor-1 alpha by direct interaction with two distinct domains of the CREB-binding protein/p300
Abstract
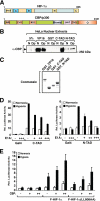
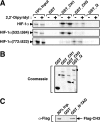
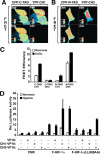
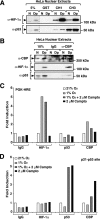
Activation of transcription in response to low oxygen tension is mediated by the hypoxia-inducible factor-1 (HIF-1). HIF-1 is a heterodimer of two proteins: aryl hydrocarbon receptor nuclear translocator and the oxygen-regulated HIF-1 alpha. The C-terminal activation domain of HIF-1 alpha has been shown to interact with cysteine/histidine-rich region 1 (CH1) of the coactivator CBP/p300 in a hypoxia-dependent manner. However, HIF forms lacking C-terminal activation domain (naturally occurring or genetically engineered) are still able to activate transcription of target genes in hypoxia. Here, we demonstrate that the N-terminal activation domain (N-TAD) of HIF-1 alpha interacts with endogenous CBP and that this interaction facilitates its transactivation function. Our results show that interaction of HIF-1 alpha N-TAD with CBP/p300 is mediated by the CH3 region of CBP known to interact with, among other factors, p53. Using fluorescence resonance energy transfer experiments, we demonstrate that N-TAD interacts with CH3 in vivo. Coimmunoprecipitation assays using endogenous proteins showed that immunoprecipitation of CBP in hypoxia results in the recovery of a larger fraction of HIF-1 alpha than of p53. Chromatin immunoprecipitation demonstrated that at 1% O(2) CBP is recruited to a HIF-1 alpha but not to a p53 target gene. Upon activation of both pathways, lower levels of chromatin-associated CBP were detected at either target gene promoter. These results identify CBP as the coactivator directly interacting with HIF-1 alpha N-TAD and mediating the transactivation function of this domain. Thus, we suggest that in hypoxia HIF-1 alpha is a major CBP-interacting transcription factor that may compete with other CBP-dependent factors, including p53, for limiting amounts of this coactivator, underscoring the complexity in the regulation of gene expression by HIF-1 alpha.
Figures
Similar articles
-
CITED2 controls the hypoxic signaling by snatching p300 from the two distinct activation domains of HIF-1α.Biochim Biophys Acta. 2011 Dec;1813(12):2008-16. doi: 10.1016/j.bbamcr.2011.08.018. Epub 2011 Sep 8. Biochim Biophys Acta. 2011. PMID: 21925214
-
Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein.J Biol Chem. 2002 Oct 11;277(41):38723-30. doi: 10.1074/jbc.M205051200. Epub 2002 Jul 19. J Biol Chem. 2002. PMID: 12133832
-
Modulation of p300 binding by posttranslational modifications of the C-terminal activation domain of hypoxia-inducible factor-1alpha.FEBS Lett. 2007 Apr 17;581(8):1542-8. doi: 10.1016/j.febslet.2007.03.015. Epub 2007 Mar 15. FEBS Lett. 2007. PMID: 17382325
-
Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300.J Biol Chem. 2016 Mar 25;291(13):6714-22. doi: 10.1074/jbc.R115.692020. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851278 Free PMC article. Review.
-
Recent Advances in the Discovery of HIF-1α-p300/CBP Inhibitors as Anti-Cancer Agents.Mini Rev Med Chem. 2018;18(4):296-309. doi: 10.2174/1389557516666160630124938. Mini Rev Med Chem. 2018. PMID: 27484627 Review.
Cited by
-
Gene regulation by histone-modifying enzymes under hypoxic conditions: a focus on histone methylation and acetylation.Exp Mol Med. 2022 Jul;54(7):878-889. doi: 10.1038/s12276-022-00812-1. Epub 2022 Jul 22. Exp Mol Med. 2022. PMID: 35869366 Free PMC article. Review.
-
Synthetic transactivation screening reveals ETV4 as broad coactivator of hypoxia-inducible factor signaling.Nucleic Acids Res. 2012 Mar;40(5):1928-43. doi: 10.1093/nar/gkr978. Epub 2011 Nov 10. Nucleic Acids Res. 2012. PMID: 22075993 Free PMC article.
-
Epigenetics: new questions on the response to hypoxia.Int J Mol Sci. 2011;12(7):4705-21. doi: 10.3390/ijms12074705. Epub 2011 Jul 21. Int J Mol Sci. 2011. PMID: 21845106 Free PMC article. Review.
-
Combination of betulinic acid and chidamide inhibits acute myeloid leukemia by suppression of the HIF1α pathway and generation of reactive oxygen species.Oncotarget. 2017 Oct 16;8(55):94743-94758. doi: 10.18632/oncotarget.21889. eCollection 2017 Nov 7. Oncotarget. 2017. PMID: 29212263 Free PMC article.
-
Histone deacetylase inhibitors: the epigenetic therapeutics that repress hypoxia-inducible factors.J Biomed Biotechnol. 2011;2011:197946. doi: 10.1155/2011/197946. Epub 2010 Dec 5. J Biomed Biotechnol. 2011. PMID: 21151670 Free PMC article. Review.
References
-
- Ruas J. L., Poellinger L. (2005) Semin. Cell Dev. Biol. 16, 514–522 - PubMed
-
- Lendahl U., Lee K. L., Yang H., Poellinger L. (2009) Nat. Rev. Genet. 10, 821–832 - PubMed
-
- Epstein A. C., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J. (2001) Cell 107, 43–54 - PubMed
-
- Bruick R. K., McKnight S. L. (2001) Science 294, 1337–1340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

