Double duty for nuclear proteins--the price of more open forms of mitosis
- PMID: 19879010
- PMCID: PMC2829850
- DOI: 10.1016/j.tig.2009.10.005
Double duty for nuclear proteins--the price of more open forms of mitosis
Abstract
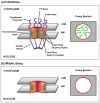
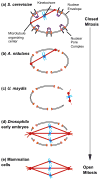
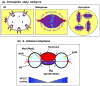
During cell division, eukaryotic cells pass on their genetic material to the next generation by undergoing mitosis, which segregates their chromosomes. During mitosis, the nuclear envelope, nuclear pore complexes and nucleolus must also be segregated. Cells achieve this in a range of different forms of mitosis, from closed, in which these nuclear structures remain intact, to open, in which these nuclear structures are disassembled. In between lies a smorgasbord of intermediate forms of mitosis, displaying varying degrees of nuclear disassembly. Gathering evidence is revealing links between the extent of nuclear disassembly and the evolution of new roles for nuclear proteins during mitosis. We propose that proteins with such double duties help coordinate reassembly of the nucleus with chromosomal segregation.
Figures

Similar articles
-
Orchestrating nuclear envelope disassembly and reassembly during mitosis.Nat Rev Mol Cell Biol. 2009 Mar;10(3):178-91. doi: 10.1038/nrm2641. Nat Rev Mol Cell Biol. 2009. PMID: 19234477 Review.
-
Nucleocytoplasmic sorting of macromolecules following mitosis: fate of nuclear constituents after inhibition of pore complex function.Eur J Cell Biol. 1989 Oct;50(1):209-19. Eur J Cell Biol. 1989. PMID: 2482180
-
Nucleolar separation from chromosomes during Aspergillus nidulans mitosis can occur without spindle forces.Mol Biol Cell. 2009 Apr;20(8):2132-45. doi: 10.1091/mbc.e08-10-1046. Epub 2009 Feb 11. Mol Biol Cell. 2009. PMID: 19211837 Free PMC article.
-
VPS72/YL1-Mediated H2A.Z Deposition Is Required for Nuclear Reassembly after Mitosis.Cells. 2020 Jul 16;9(7):1702. doi: 10.3390/cells9071702. Cells. 2020. PMID: 32708675 Free PMC article.
-
[The relationship between nuclear-cytoplasmic transport and chromosome segregation].Tsitologiia. 2005;47(3):263-76. Tsitologiia. 2005. PMID: 16706172 Review. Russian.
Cited by
-
Restraint of the G2/M transition by the SR/RRM family mRNA shuttling binding protein SNXAHRB1 in Aspergillus nidulans.Genetics. 2014 Oct;198(2):617-33. doi: 10.1534/genetics.114.167445. Epub 2014 Aug 7. Genetics. 2014. PMID: 25104516 Free PMC article.
-
Aspergillus SUMOylation mutants exhibit chromosome segregation defects including chromatin bridges.Genetics. 2023 Dec 6;225(4):iyad169. doi: 10.1093/genetics/iyad169. Genetics. 2023. PMID: 37724751 Free PMC article.
-
Insights into dynamic mitotic chromatin organization through the NIMA kinase suppressor SonC, a chromatin-associated protein involved in the DNA damage response.Genetics. 2014 Jan;196(1):177-95. doi: 10.1534/genetics.113.156745. Epub 2013 Nov 8. Genetics. 2014. PMID: 24214344 Free PMC article.
-
Nup2 requires a highly divergent partner, NupA, to fulfill functions at nuclear pore complexes and the mitotic chromatin region.Mol Biol Cell. 2015 Feb 15;26(4):605-21. doi: 10.1091/mbc.E14-09-1359. Epub 2014 Dec 24. Mol Biol Cell. 2015. PMID: 25540430 Free PMC article.
-
The spindle matrix protein, Chromator, is a novel tubulin binding protein that can interact with both microtubules and free tubulin.PLoS One. 2014 Jul 29;9(7):e103855. doi: 10.1371/journal.pone.0103855. eCollection 2014. PLoS One. 2014. PMID: 25072297 Free PMC article.
References
-
- Heath IB. Variant mitoses in lower eukaryotes: Indicators of the evolution of mitosis? Int Rev Cytol. 1980;64:1–80. - PubMed
-
- Paddy MR, et al. Time-resolved, in vivo studies of mitotic spindle formation and nuclear lamina breakdown in Drosophila early embryos. J Cell Sci. 1996;109:591–607. - PubMed
-
- Kiseleva E, et al. Steps of nuclear pore complex disassembly and reassembly during mitosis in early Drosophila embryos. J Cell Sci. 2001;114:3607–3618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

