Sensation and signaling of alpha-ketoglutarate and adenylylate energy charge by the Escherichia coli PII signal transduction protein require cooperation of the three ligand-binding sites within the PII trimer
- PMID: 19877670
- PMCID: PMC2786245
- DOI: 10.1021/bi9011594
Sensation and signaling of alpha-ketoglutarate and adenylylate energy charge by the Escherichia coli PII signal transduction protein require cooperation of the three ligand-binding sites within the PII trimer
Abstract
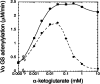
PII proteins are sensors of alpha-ketoglutarate and adenylylate energy charge that regulate signal transduction proteins, metabolic enzymes, and permeases involved in nitrogen assimilation. Here, purified Escherichia coli PII and two of its receptors, ATase and NRII, were used to study the mechanisms of sensation by PII. We assembled heterotrimeric forms of PII from wild-type and mutant subunits, which allowed us to assess the role of the three binding sites for alpha-ketoglutarate and adenylylate nucleotide in the PII trimer. Signaling of alpha-ketoglutarate and adenylylate energy charge by these heterotrimeric PII proteins required multiple binding sites for these effectors, and the ligand-binding sites on different subunits could influence the function of a single subunit interacting with a receptor, implying communication between PII subunits. Wild-type and heterotrimeric forms of PII were also used to examine the effects of alpha-ketoglutarate and ADP on PII activation of the adenylyltransferase (AT) activity of ATase. Previous work showed that when ATP was the sole adenylylate nucleotide, alpha-ketoglutarate controlled the extent of PII activation but did not alter the PII activation constant (K(act)). We show that ADP affected both the PII K(act) and the extent of activation by PII. When ATP was present, ADP dramatically reduced the K(act) for wild-type PII, and this effect was antagonized by alpha-ketoglutarate. Consequently, when ATP was present, the antagonism between ADP and alpha-ketoglutarate allowed each of these effectors to influence the PII K(act) for activation of ATase. A study of heterotrimeric forms of PII suggested that the major part of the ability of ADP to improve the binding of PII to ATase required multiple nucleotide binding sites and intersubunit communication. We also used nondenaturing gel electrophoresis to investigate the effect of ADP and alpha-ketoglutarate on the binding of PII to ATase and NRII. These studies showed that ATase and NRII differ in their requirements for interaction with PII, and that under the appropriate conditions, the antagonism between alpha-ketoglutarate and ADP allowed each of these effectors to influence the binding of PII to receptors.
Figures
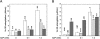




Similar articles
-
Alpha-ketoglutarate controls the ability of the Escherichia coli PII signal transduction protein to regulate the activities of NRII (NrB but does not control the binding of PII to NRII.Biochemistry. 2009 Dec 8;48(48):11514-21. doi: 10.1021/bi901158h. Biochemistry. 2009. PMID: 19877669 Free PMC article.
-
Escherichia coli PII signal transduction protein controlling nitrogen assimilation acts as a sensor of adenylate energy charge in vitro.Biochemistry. 2007 Nov 13;46(45):12979-96. doi: 10.1021/bi701062t. Epub 2007 Oct 16. Biochemistry. 2007. PMID: 17939683
-
Probing interactions of the homotrimeric PII signal transduction protein with its receptors by use of PII heterotrimers formed in vitro from wild-type and mutant subunits.J Bacteriol. 1997 Jul;179(13):4354-60. doi: 10.1128/jb.179.13.4354-4360.1997. J Bacteriol. 1997. PMID: 9209054 Free PMC article.
-
Global carbon/nitrogen control by PII signal transduction in cyanobacteria: from signals to targets.FEMS Microbiol Rev. 2004 Jun;28(3):319-33. doi: 10.1016/j.femsre.2003.11.001. FEMS Microbiol Rev. 2004. PMID: 15449606 Review.
-
PII signal transduction proteins: sensors of alpha-ketoglutarate that regulate nitrogen metabolism.Curr Opin Microbiol. 2005 Apr;8(2):168-73. doi: 10.1016/j.mib.2005.02.011. Curr Opin Microbiol. 2005. PMID: 15802248 Review.
Cited by
-
Phosphonate analogs of 2-oxoglutarate perturb metabolism and gene expression in illuminated Arabidopsis leaves.Front Plant Sci. 2012 Jun 4;3:114. doi: 10.3389/fpls.2012.00114. eCollection 2012. Front Plant Sci. 2012. PMID: 22876250 Free PMC article.
-
Post-translational modification of P II signal transduction proteins.Front Microbiol. 2015 Jan 6;5:763. doi: 10.3389/fmicb.2014.00763. eCollection 2014. Front Microbiol. 2015. PMID: 25610437 Free PMC article. Review.
-
GlnK Facilitates the Dynamic Regulation of Bacterial Nitrogen Assimilation.Biophys J. 2017 May 23;112(10):2219-2230. doi: 10.1016/j.bpj.2017.04.012. Biophys J. 2017. PMID: 28538158 Free PMC article.
-
Dual positive and negative control of Chlamydomonas PII signal transduction protein expression by nitrate/nitrite and NO via the components of nitric oxide cycle.BMC Plant Biol. 2018 Nov 27;18(1):305. doi: 10.1186/s12870-018-1540-x. BMC Plant Biol. 2018. PMID: 30482162 Free PMC article.
-
The Emergence of 2-Oxoglutarate as a Master Regulator Metabolite.Microbiol Mol Biol Rev. 2015 Dec;79(4):419-35. doi: 10.1128/MMBR.00038-15. Microbiol Mol Biol Rev. 2015. PMID: 26424716 Free PMC article. Review.
References
-
- Ninfa A. J.; Atkinson M. R. (2000) PII signal transduction proteins. Trends Microbiol. 8, 172–179. - PubMed
-
- Sant’Anna F. H.; Trentini D. B.; de Souto Weber S.; Cecago R.; Ceroni de Silva S.; Schrank I. S. (2009) The PII superfamily revisited: A novel group and evolutionary insights. J. Mol. Evol. 68, 322–336. - PubMed
-
- Ninfa A. J.; Jiang P. (2005) PII signal transduction proteins: Sensors of α-ketoglutarate that regulate nitrogen metabolism. Curr. Opin. Microbiol. 8, 168–173. - PubMed
-
- Forchhammer K. (2008) PII signal transducers: Novel functional and structural insights. Trends Microbiol. 16, 65–72. - PubMed
-
- Kamberov E. S.; Atkinson M. R.; Ninfa A. J. (1995) The Escherichia coli PII signal transduction protein is activated upon binding 2-ketoglutarate and ATP. J. Biol. Chem. 270, 17797–17807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

