The cellular architecture of the larval zebrafish tectum, as revealed by gal4 enhancer trap lines
- PMID: 19862330
- PMCID: PMC2763897
- DOI: 10.3389/neuro.04.013.2009
The cellular architecture of the larval zebrafish tectum, as revealed by gal4 enhancer trap lines
Abstract
We have carried out a Gal4 enhancer trap screen in zebrafish, and have generated 184 stable transgenic lines with interesting expression patterns throughout the nervous system. Of these, three display clear expression in the tectum, each with a distinguishable and stereotyped distribution of Gal4 expressing cells. Detailed morphological analysis of single cells, using a genetic "Golgi-like" labelling method, revealed four common cell types (superficial, periventricular, shallow periventricular, and radial glial), along with a range of other less common neurons. The shallow periventricular (PV) and a subset of the PV neurons are tectal efferent neurons that target various parts of the reticular formation. We find that it is specifically PV neurons with dendrites in the deep tectal neuropil that target the reticular formation. This indicates that these neurons receive the tectum's highly processed visual information (which is fed from the superficial retinorecipient layers), and relay it to premotor regions. Our results show that the larval tectum, both broadly and at the single cell level, strongly resembles a miniature version of its adult counterpart, and that it has all of the necessary anatomical characteristics to inform motor responses based on sensory input. We also demonstrate that mosaic expression of GFP in Gal4 enhancer trap lines can be used to describe the types and abundance of cells in an expression pattern, including the architectures of individual neurons. Such detailed anatomical descriptions will be an important part of future efforts to describe the functions of discrete tectal circuits in the generation of behavior.
Keywords: Gal4/UAS; anatomy; neuron; periventricular; single-cell morphology; tectum; transgenics; zebrafish.
Figures
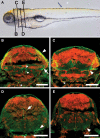
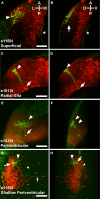

Similar articles
-
Hypothalamic Projections to the Optic Tectum in Larval Zebrafish.Front Neuroanat. 2018 Jan 17;11:135. doi: 10.3389/fnana.2017.00135. eCollection 2017. Front Neuroanat. 2018. PMID: 29403362 Free PMC article.
-
Neuron types in the zebrafish optic tectum labeled by an id2b transgene.J Comp Neurol. 2020 May;528(7):1173-1188. doi: 10.1002/cne.24815. Epub 2019 Nov 26. J Comp Neurol. 2020. PMID: 31725916
-
The Tol2-mediated Gal4-UAS method for gene and enhancer trapping in zebrafish.Methods. 2009 Nov;49(3):275-81. doi: 10.1016/j.ymeth.2009.01.004. Epub 2009 Feb 3. Methods. 2009. PMID: 19835787 Free PMC article.
-
Roles of periventricular neurons in retinotectal transmission in the optic tectum.Prog Neurobiol. 2006 Jun;79(2):112-21. doi: 10.1016/j.pneurobio.2006.06.002. Epub 2006 Aug 9. Prog Neurobiol. 2006. PMID: 16901616 Review.
-
Gal4 Driver Transgenic Zebrafish: Powerful Tools to Study Developmental Biology, Organogenesis, and Neuroscience.Adv Genet. 2016;95:65-87. doi: 10.1016/bs.adgen.2016.04.002. Epub 2016 Jun 13. Adv Genet. 2016. PMID: 27503354 Review.
Cited by
-
New tools for the identification of developmentally regulated enhancer regions in embryonic and adult zebrafish.Zebrafish. 2013 Mar;10(1):21-9. doi: 10.1089/zeb.2012.0775. Epub 2013 Mar 5. Zebrafish. 2013. PMID: 23461416 Free PMC article.
-
Single-Cell RNA Sequencing Characterizes the Molecular Heterogeneity of the Larval Zebrafish Optic Tectum.Front Mol Neurosci. 2022 Feb 10;15:818007. doi: 10.3389/fnmol.2022.818007. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35221915 Free PMC article.
-
Investigating the genetics of visual processing, function and behaviour in zebrafish.Neurogenetics. 2011 May;12(2):97-116. doi: 10.1007/s10048-011-0273-x. Epub 2011 Jan 26. Neurogenetics. 2011. PMID: 21267617 Review.
-
Hypothalamic Projections to the Optic Tectum in Larval Zebrafish.Front Neuroanat. 2018 Jan 17;11:135. doi: 10.3389/fnana.2017.00135. eCollection 2017. Front Neuroanat. 2018. PMID: 29403362 Free PMC article.
-
Delaying Gal4-driven gene expression in the zebrafish with morpholinos and Gal80.PLoS One. 2011 Jan 26;6(1):e16587. doi: 10.1371/journal.pone.0016587. PLoS One. 2011. PMID: 21298067 Free PMC article.
References
-
- Allen Brain Atlas Available at: http://www.brain-map.org/
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

