Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines
- PMID: 19861464
- PMCID: PMC2783346
- DOI: 10.1158/1078-0432.CCR-09-1067
Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines
Abstract
Purpose: A variety of cancers, including malignant gliomas, overexpress transforming growth factor-beta (TGF-beta), which helps tumors evade effective immune surveillance through a variety of mechanisms, including inhibition of CD8(+) CTLs and enhancing the generation of regulatory T (T(reg)) cells. We hypothesized that inhibition of TGF-beta would improve the efficacy of vaccines targeting glioma-associated antigen (GAA)-derived CTL epitopes by reversal of immunosuppression.
Experimental design: Mice bearing orthotopic GL261 gliomas were treated systemically with a TGF-beta-neutralizing monoclonal antibody, 1D11, with or without s.c. vaccinations of synthetic peptides for GAA-derived CTL epitopes, GARC-1 (77-85) and EphA2 (671-679), emulsified in incomplete Freund's adjuvant.
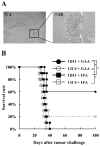
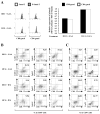
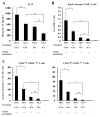
Results: Mice receiving the combination regimen exhibited significantly prolonged survival compared with mice receiving either 1D11 alone, GAA vaccines alone, or mock treatments alone. TGF-beta neutralization enhanced the systemic induction of antigen-specific CTLs in glioma-bearing mice. Flow cytometric analyses of brain-infiltrating lymphocytes revealed that 1D11 treatment suppressed phosphorylation of Smad2, increased GAA-reactive/IFN-gamma-producing CD8(+) T cells, and reduced CD4(+)/FoxP3(+) T(reg) cells in the glioma microenvironment. Neutralization of TGF-beta also upregulated plasma levels of interleukin-12, macrophage inflammatory protein-1 alpha, and IFN-inducible protein-10, suggesting a systemic promotion of type-1 cytokine/chemokine production. Furthermore, 1D11 treatment upregulated plasma interleukin-15 levels and promoted the persistence of GAA-reactive CD8(+) T cells in glioma-bearing mice.
Conclusions: These data suggest that systemic inhibition of TGF-beta by 1D11 can reverse the suppressive immunologic environment of orthotopic tumor-bearing mice both systemically and locally, thereby enhancing the therapeutic efficacy of GAA vaccines.
Figures

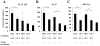
Comment in
-
Enhancing cancer vaccine efficacy via modulation of the tumor microenvironment.Clin Cancer Res. 2009 Nov 1;15(21):6476-8. doi: 10.1158/1078-0432.CCR-09-2256. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861446
Similar articles
-
Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody.Clin Cancer Res. 2009 Nov 1;15(21):6560-9. doi: 10.1158/1078-0432.CCR-09-1066. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861451 Free PMC article.
-
Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma.J Immunol. 2008 Feb 15;180(4):2089-98. doi: 10.4049/jimmunol.180.4.2089. J Immunol. 2008. PMID: 18250414
-
Tumor localization of an anti-TGF-β antibody and its effects on gliomas.Int J Oncol. 2011 Jan;38(1):51-9. Int J Oncol. 2011. PMID: 21109925
-
Brain tumor immunotherapy with type-1 polarizing strategies.Ann N Y Acad Sci. 2009 Sep;1174:18-23. doi: 10.1111/j.1749-6632.2009.04932.x. Ann N Y Acad Sci. 2009. PMID: 19769732 Review.
-
The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain.Brain Res Brain Res Rev. 1995 Sep;21(2):128-51. doi: 10.1016/0165-0173(95)00010-0. Brain Res Brain Res Rev. 1995. PMID: 8866671 Review.
Cited by
-
A positive-margin resection model recreates the postsurgical tumor microenvironment and is a reliable model for adjuvant therapy evaluation.Cancer Biol Ther. 2012 Jul;13(9):745-55. doi: 10.4161/cbt.20557. Epub 2012 May 23. Cancer Biol Ther. 2012. PMID: 22617772 Free PMC article.
-
Chloroquine inhibits the malignant phenotype of glioblastoma partially by suppressing TGF-beta.Invest New Drugs. 2015 Oct;33(5):1020-31. doi: 10.1007/s10637-015-0275-x. Epub 2015 Aug 15. Invest New Drugs. 2015. PMID: 26271735
-
Can inhibition of angiogenesis and stimulation of immune response be combined into a more effective antitumor therapy?Cancer Immunol Immunother. 2010 Oct;59(10):1449-55. doi: 10.1007/s00262-010-0873-6. Epub 2010 Jun 16. Cancer Immunol Immunother. 2010. PMID: 20552191 Free PMC article. Review.
-
Synthetic High-density Lipoprotein Nanodiscs for Personalized Immunotherapy Against Gliomas.Clin Cancer Res. 2020 Aug 15;26(16):4369-4380. doi: 10.1158/1078-0432.CCR-20-0341. Epub 2020 May 21. Clin Cancer Res. 2020. PMID: 32439701 Free PMC article.
-
Strategies to improve the immunogenicity of anticancer vaccines based on dendritic cell/malignant cell fusions.Oncoimmunology. 2013 Sep 1;2(9):e25994. doi: 10.4161/onci.25994. Epub 2013 Sep 12. Oncoimmunology. 2013. PMID: 24228229 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Eguchi J, Hatano M, Nishimura F, Zhu X, Dusak JE, Sato H, et al. Identification of interleukin-13 receptor alpha2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. - PubMed
-
- Ueda R, Kinoshita E, Ito R, Kawase T, Kawakami Y, Toda M. Induction of protective and therapeutic antitumor immunity by a DNA vaccine with a glioma antigen, SOX6. Int J Cancer. 2008;122:2274–2279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

