Alternate strategies of Hsp90 modulation for the treatment of cancer and other diseases
- PMID: 19860731
- PMCID: PMC2853258
- DOI: 10.2174/156802609789895683
Alternate strategies of Hsp90 modulation for the treatment of cancer and other diseases
Abstract
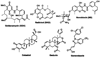
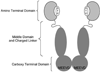
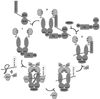
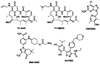
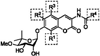
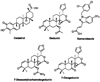
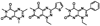
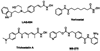
The 90 kDa heat shock protein (Hsp90) has become a validated target for the development of anti-cancer agents. Several Hsp90 inhibitors are currently under clinical trial investigation for the treatment of cancer. All of these agents inhibit Hsp90's protein folding activity by binding to the N-terminal ATP binding site of the Hsp90 molecular chaperone. Administration of these investigational drugs elicits induction of the heat shock response, or the overexpression of several Hsps, which exhibit antiapoptotic and pro-survival effects that may complicate the application of these inhibitors. To circumvent this issue, alternate mechanisms for Hsp90 inhibition that do not elicit the heat shock response have been identified and pursued. After providing background on the structure, function, and mechanism of the Hsp90 protein folding machinery, this review describes several mechanisms of Hsp90 modulation via small molecules that do not induce the heat shock response.
Figures
Similar articles
-
Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket.Curr Med Chem. 2008;15(26):2702-17. doi: 10.2174/092986708786242895. Curr Med Chem. 2008. PMID: 18991631 Free PMC article. Review.
-
Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects.Adv Cancer Res. 2016;129:51-88. doi: 10.1016/bs.acr.2015.12.001. Epub 2016 Feb 10. Adv Cancer Res. 2016. PMID: 26916001 Free PMC article. Review.
-
Strategies for stalling malignancy: targeting cancer's addiction to Hsp90.Curr Top Med Chem. 2009;9(15):1352-68. doi: 10.2174/156802609789895656. Curr Top Med Chem. 2009. PMID: 19860736 Review.
-
Assays for identification of Hsp90 inhibitors and biochemical methods for discriminating their mechanism of action.Curr Top Med Chem. 2009;9(15):1462-78. doi: 10.2174/156802609789895692. Curr Top Med Chem. 2009. PMID: 19860729 Review.
-
Dipyridamole interacts with the N-terminal domain of HSP90 and antagonizes the function of the chaperone in multiple cancer cell lines.Biochem Pharmacol. 2023 Jan;207:115376. doi: 10.1016/j.bcp.2022.115376. Epub 2022 Dec 10. Biochem Pharmacol. 2023. PMID: 36513142
Cited by
-
Natural Product Inspired N-Terminal Hsp90 Inhibitors: From Bench to Bedside?Med Res Rev. 2016 Jan;36(1):92-118. doi: 10.1002/med.21351. Epub 2015 May 25. Med Res Rev. 2016. PMID: 26010985 Free PMC article. Review.
-
A novel function of novobiocin: disrupting the interaction of HIF 1α and p300/CBP through direct binding to the HIF1α C-terminal activation domain.PLoS One. 2013 May 6;8(5):e62014. doi: 10.1371/journal.pone.0062014. Print 2013. PLoS One. 2013. PMID: 23671581 Free PMC article.
-
Heat Shock Protein 90 (HSP90) Inhibitors as Anticancer Medicines: A Review on the Computer-Aided Drug Discovery Approaches over the Past Five Years.Comput Math Methods Med. 2022 May 31;2022:2147763. doi: 10.1155/2022/2147763. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35685897 Free PMC article. Review.
-
Development of Phenyl Cyclohexylcarboxamides as a Novel Class of Hsp90 C-terminal Inhibitors.Chemistry. 2017 Nov 21;23(65):16574-16585. doi: 10.1002/chem.201703206. Epub 2017 Nov 3. Chemistry. 2017. PMID: 28940589 Free PMC article.
-
Design of Allosteric Stimulators of the Hsp90 ATPase as New Anticancer Leads.Chemistry. 2017 Apr 19;23(22):5188-5192. doi: 10.1002/chem.201700169. Epub 2017 Mar 22. Chemistry. 2017. PMID: 28207175 Free PMC article.
References
-
- Paul S. Dysfunction of the ubiquitin-proteasome system in multiple disease conditions: therapeutic approaches. Bioessays. 2008;30:1172–1184. - PubMed
-
- Messaed C, Rouleau GA. Molecular mechanisms underlying polyalanine diseases. Neurobiol. Dis. 2009;34:397–405. - PubMed
-
- Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid. Redox. Signal. 2008;10:1713–1753. - PubMed
-
- Vardiman JW. Chronic myelogenous leukemia, BCR-ABL1+ Am. J. Clin. Pathol. 2009;132:250–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

