New strategies for the design of folded peptoids revealed by a survey of noncovalent interactions in model systems
- PMID: 19860427
- PMCID: PMC3175426
- DOI: 10.1021/ja907184g
New strategies for the design of folded peptoids revealed by a survey of noncovalent interactions in model systems
Abstract
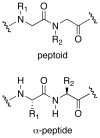
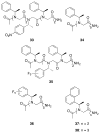
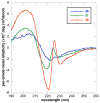
Controlling the equilibria between backbone cis- and trans-amides in peptoids, or N-substituted glycine oligomers, constitutes a significant challenge in the construction of discretely folded peptoid structures. Through the analysis of a set of monomeric peptoid model systems, we have developed new and general strategies for controlling peptoid conformation that utilize local noncovalent interactions to regulate backbone amide rotameric equilibria, including n-->pi*, steric, and hydrogen bonding interactions. The chemical functionalities required to implement these strategies are typically confined to the peptoid side chains, preserve chirality at the side chain N-alpha-carbon known to engender peptoid structure, and are fully compatible with standard peptoid synthesis techniques. Our examinations of peptoid model systems have also elucidated how solvents affect various side chain-backbone interactions, revealing fundamental aspects of these noncovalent interactions in peptoids that were largely uncharacterized previously. As validation of our monomeric model systems, we extended the scope of this study to include peptoid oligomers and have now demonstrated the importance of local steric and n-->pi* interactions in dictating the structures of larger, folded peptoids. This new, modular design strategy has guided the construction of peptoids containing 1-naphthylethyl side chains, which we show can be utilized to effectively eliminate trans-amide rotamers from the peptoid backbone, yielding the most conformationally homogeneous class of peptoid structures yet reported in terms of amide rotamerism. Overall, this research has afforded a valuable and expansive set of design tools for the construction of both discretely folded peptoids and structurally biased peptoid libraries and should shape our understanding of peptoid folding.
Figures


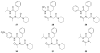
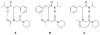
Similar articles
-
Construction of peptoids with all trans-amide backbones and peptoid reverse turns via the tactical incorporation of N-aryl side chains capable of hydrogen bonding.J Org Chem. 2010 Sep 17;75(18):6068-78. doi: 10.1021/jo101075a. J Org Chem. 2010. PMID: 20722367 Free PMC article.
-
Tuning peptoid secondary structure with pentafluoroaromatic functionality: a new design paradigm for the construction of discretely folded peptoid structures.J Am Chem Soc. 2006 Nov 8;128(44):14378-87. doi: 10.1021/ja065248o. J Am Chem Soc. 2006. PMID: 17076512
-
Extraordinarily robust polyproline type I peptoid helices generated via the incorporation of α-chiral aromatic N-1-naphthylethyl side chains.J Am Chem Soc. 2011 Oct 5;133(39):15559-67. doi: 10.1021/ja204755p. Epub 2011 Sep 13. J Am Chem Soc. 2011. PMID: 21861531 Free PMC article.
-
Strategies to Control the Cis-Trans Isomerization of Peptoid Amide Bonds.Chem Asian J. 2022 Jun 1;17(11):e202200149. doi: 10.1002/asia.202200149. Epub 2022 Apr 20. Chem Asian J. 2022. PMID: 35362652 Review.
-
Structure-function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function.Org Biomol Chem. 2009 Apr 21;7(8):1508-24. doi: 10.1039/b817980h. Epub 2009 Feb 11. Org Biomol Chem. 2009. PMID: 19343235 Free PMC article. Review.
Cited by
-
n→π* interactions engender chirality in carbonyl groups.Org Lett. 2014 Jul 3;16(13):3421-3. doi: 10.1021/ol5012967. Epub 2014 Jun 13. Org Lett. 2014. PMID: 24926562 Free PMC article.
-
Peptoid Residues Make Diverse, Hyperstable Collagen Triple-Helices.J Am Chem Soc. 2021 Jul 28;143(29):10910-10919. doi: 10.1021/jacs.1c00708. Epub 2021 Jul 13. J Am Chem Soc. 2021. PMID: 34255504 Free PMC article.
-
Systematic Investigation on Acid-Catalyzed Truncation of N-Acylated Peptoids.Int J Mol Sci. 2024 Oct 23;25(21):11390. doi: 10.3390/ijms252111390. Int J Mol Sci. 2024. PMID: 39518946 Free PMC article.
-
Synthesis of libraries of peptidomimetic compounds containing a 2-oxopiperazine unit in the main chain.Org Biomol Chem. 2013 Apr 7;11(13):2088-92. doi: 10.1039/c3ob27476d. Org Biomol Chem. 2013. PMID: 23440085 Free PMC article.
-
Intimate interactions with carbonyl groups: dipole-dipole or n→π*?J Org Chem. 2013 Mar 1;78(5):2099-103. doi: 10.1021/jo302265k. Epub 2012 Dec 10. J Org Chem. 2013. PMID: 23163960 Free PMC article.
References
-
- Figliozzi GM, Goldsmith R, Ng SC, Banville SC, Zuckermann RN. Methods Enzymol. 1996;267:437–447. - PubMed
-
- Zuckermann RN, Kerr JM, Kent SBH, Moos WH. J Am Chem Soc. 1992;114:10646–10647.
-
- Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH. Bioorg Med Chem Lett. 1994;4:2657–2662.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

