Wnt-Ror signaling to SIA and SIB neurons directs anterior axon guidance and nerve ring placement in C. elegans
- PMID: 19855022
- PMCID: PMC2861721
- DOI: 10.1242/dev.038109
Wnt-Ror signaling to SIA and SIB neurons directs anterior axon guidance and nerve ring placement in C. elegans
Abstract
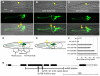
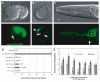
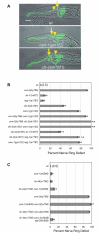
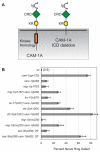
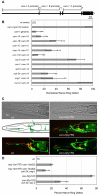
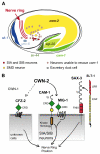
Wnt signaling through Frizzled proteins guides posterior cells and axons in C. elegans into different spatial domains. Here we demonstrate an essential role for Wnt signaling through Ror tyrosine kinase homologs in the most prominent anterior neuropil, the nerve ring. A genetic screen uncovered cwn-2, the C. elegans homolog of Wnt5, as a regulator of nerve ring placement. In cwn-2 mutants, all neuronal structures in and around the nerve ring are shifted to an abnormal anterior position. cwn-2 is required at the time of nerve ring formation; it is expressed by cells posterior of the nerve ring, but its precise site of expression is not critical for its function. In nerve ring development, cwn-2 acts primarily through the Wnt receptor CAM-1 (Ror), together with the Frizzled protein MIG-1, with parallel roles for the Frizzled protein CFZ-2. The identification of CAM-1 as a CWN-2 receptor contrasts with CAM-1 action as a non-receptor in other C. elegans Wnt pathways. Cell-specific rescue of cam-1 and cell ablation experiments reveal a crucial role for the SIA and SIB neurons in positioning the nerve ring, linking Wnt signaling to specific cells that organize the anterior nervous system.
Figures


Similar articles
-
A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans.PLoS Genet. 2010 Aug 12;6(8):e1001056. doi: 10.1371/journal.pgen.1001056. PLoS Genet. 2010. PMID: 20711352 Free PMC article.
-
The conserved transmembrane RING finger protein PLR-1 downregulates Wnt signaling by reducing Frizzled, Ror and Ryk cell-surface levels in C. elegans.Development. 2014 Feb;141(3):617-28. doi: 10.1242/dev.101600. Epub 2014 Jan 8. Development. 2014. PMID: 24401370 Free PMC article.
-
Regulation of Axon Guidance by the Wnt Receptor Ror/CAM-1 in the PVT Guidepost Cell in Caenorhabditis elegans.Genetics. 2017 Dec;207(4):1533-1545. doi: 10.1534/genetics.117.300375. Epub 2017 Oct 9. Genetics. 2017. PMID: 28993416 Free PMC article.
-
Should I stay or should I go: Wnt signals at the synapse.Cell. 2007 Aug 24;130(4):593-6. doi: 10.1016/j.cell.2007.08.007. Cell. 2007. PMID: 17719537 Review.
-
Ror receptor tyrosine kinases: orphans no more.Trends Cell Biol. 2008 Nov;18(11):536-44. doi: 10.1016/j.tcb.2008.08.006. Epub 2008 Oct 9. Trends Cell Biol. 2008. PMID: 18848778 Free PMC article. Review.
Cited by
-
Neuronal target identification requires AHA-1-mediated fine-tuning of Wnt signaling in C. elegans.PLoS Genet. 2013 Jun;9(6):e1003618. doi: 10.1371/journal.pgen.1003618. Epub 2013 Jun 27. PLoS Genet. 2013. PMID: 23825972 Free PMC article.
-
Cross-modality synthesis of EM time series and live fluorescence imaging.Elife. 2022 Jun 6;11:e77918. doi: 10.7554/eLife.77918. Elife. 2022. PMID: 35666127 Free PMC article.
-
Wnt signaling through the Ror receptor in the nervous system.Mol Neurobiol. 2014 Feb;49(1):303-15. doi: 10.1007/s12035-013-8520-9. Epub 2013 Aug 30. Mol Neurobiol. 2014. PMID: 23990374 Review.
-
ngn-1/neurogenin Activates Transcription of Multiple Terminal Selector Transcription Factors in the Caenorhabditis elegans Nervous System.G3 (Bethesda). 2020 Jun 1;10(6):1949-1962. doi: 10.1534/g3.120.401126. G3 (Bethesda). 2020. PMID: 32273286 Free PMC article.
-
The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic C. elegans Sensory/Inter/Motor Neuron-Type.PLoS Genet. 2015 Aug 25;11(8):e1005480. doi: 10.1371/journal.pgen.1005480. eCollection 2015 Aug. PLoS Genet. 2015. PMID: 26305787 Free PMC article.
References
-
- Aurelio, O., Hall, D. H. and Hobert, O. (2002). Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science 295, 686-690. - PubMed
-
- Benard, C. Y., Boyanov, A., Hall, D. H. and Hobert, O. (2006). DIG-1, a novel giant protein, non-autonomously mediates maintenance of nervous system architecture. Development 133, 3329-3340. - PubMed
-
- Billiard, J., Way, D. S., Seestaller-Wehr, L. M., Moran, R. A., Mangine, A. and Bodine, P. V. (2005). The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol. Endocrinol. 19, 90-101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

