Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis
- PMID: 19838304
- PMCID: PMC2760134
- DOI: 10.1371/journal.pone.0007501
Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis
Abstract
In mammals, germ cells within the developing gonad follow a sexually dimorphic pathway. Germ cells in the murine ovary enter meiotic prophase during embryogenesis, whereas germ cells in the embryonic testis arrest in G0 of mitotic cell cycle and do not enter meiosis until after birth. In mice, retinoic acid (RA) signaling has been implicated in controlling entry into meiosis in germ cells, as meiosis in male embryonic germ cells is blocked by the activity of a RA-catabolizing enzyme, CYP26B1. However, the mechanisms regulating mitotic arrest in male germ cells are not well understood. Cyp26b1 expression in the testes begins in somatic cells at embryonic day (E) 11.5, prior to mitotic arrest, and persists throughout fetal development. Here, we show that Sertoli cell-specific loss of CYP26B1 activity between E15.5 and E16.5, several days after germ cell sex determination, causes male germ cells to exit from G0, re-enter the mitotic cell cycle and initiate meiotic prophase. These results suggest that male germ cells retain the developmental potential to differentiate in meiosis until at least at E15.5. CYP26B1 in Sertoli cells acts as a masculinizing factor to arrest male germ cells in the G0 phase of the cell cycle and prevents them from entering meiosis, and thus is essential for the maintenance of the undifferentiated state of male germ cells during embryonic development.
Conflict of interest statement
Figures


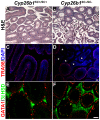


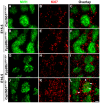

Similar articles
-
Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad.PLoS One. 2011;6(6):e20249. doi: 10.1371/journal.pone.0020249. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21674038 Free PMC article.
-
Male differentiation of germ cells induced by embryonic age-specific Sertoli cells in mice.Biol Reprod. 2012 Apr 12;86(4):112. doi: 10.1095/biolreprod.111.095943. Print 2012 Apr. Biol Reprod. 2012. PMID: 22262692 Free PMC article.
-
New testicular mechanisms involved in the prevention of fetal meiotic initiation in mice.Dev Biol. 2010 Oct 15;346(2):320-30. doi: 10.1016/j.ydbio.2010.08.002. Epub 2010 Aug 11. Dev Biol. 2010. PMID: 20707993
-
Cell cycle regulation for meiosis in mammalian germ cells.J Reprod Dev. 2023 Jun 6;69(3):139-146. doi: 10.1262/jrd.2023-010. Epub 2023 Mar 17. J Reprod Dev. 2023. PMID: 36927827 Free PMC article. Review.
-
Retinoic acid signaling in regulation of meiosis during embryonic development in mice.Genesis. 2019 Jul;57(7-8):e23327. doi: 10.1002/dvg.23327. Epub 2019 Jul 17. Genesis. 2019. PMID: 31313882 Review.
Cited by
-
The molecular and cellular basis of gonadal sex reversal in mice and humans.Wiley Interdiscip Rev Dev Biol. 2012 Jul-Aug;1(4):559-77. doi: 10.1002/wdev.42. Epub 2012 Feb 28. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801533 Free PMC article. Review.
-
Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence.Dev Biol. 2013 May 1;377(1):188-201. doi: 10.1016/j.ydbio.2013.01.031. Epub 2013 Feb 4. Dev Biol. 2013. PMID: 23391689 Free PMC article.
-
Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases.Pharmacol Ther. 2019 Dec;204:107400. doi: 10.1016/j.pharmthera.2019.107400. Epub 2019 Aug 13. Pharmacol Ther. 2019. PMID: 31419517 Free PMC article. Review.
-
Differential responsiveness of spermatogonia to retinoic acid dictates precocious differentiation but not meiotic entry during steady-state spermatogenesis†.Biol Reprod. 2023 May 10;108(5):822-836. doi: 10.1093/biolre/ioad010. Biol Reprod. 2023. PMID: 36708226 Free PMC article.
-
FBXO38 Ubiquitin Ligase Controls Sertoli Cell Maturation.Front Cell Dev Biol. 2022 Jun 13;10:914053. doi: 10.3389/fcell.2022.914053. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35769260 Free PMC article.
References
-
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. - PubMed
-
- Pennimpede T, Cameron D, Petkovich M. Regulation of murine embryonic patterning and morphogenesis by retinoic acid signaling. Adv Dev Biol. 2006;16:66–104.
-
- White JC, Highland M, Kaiser M, Clagett-Dame M. Vitamin A deficiency results in the dose-dependent acquisition of anterior character and shortening of the caudal hindbrain of the rat embryo. Dev Biol. 2000;220:263–284. - PubMed
-
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post- implantation development [see comments]. Nat Genet. 1999;21:444–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

