Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer
- PMID: 19838011
- PMCID: PMC3621568
- DOI: 10.2183/pjab.85.314
Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer
Abstract
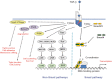
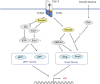
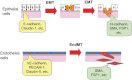
Transforming growth factor-beta (TGF-beta) is a multifunctional cytokine that induces growth arrest, tissue fibrosis, and epithelial-mesenchymal transition (EMT) through activation of Smad and non-Smad signaling pathways. EMT is the differentiation switch by which polarized epithelial cells differentiate into contractile and motile mesenchymal cells. Cell motility and invasive capacity are activated upon EMT. Multiple transcription factors, including deltaEF1/ZEB1, SIP1/ZEB2, and Snail/SNAI1, are induced by TGF-beta-Smad signaling and play critical roles in TGF-beta-induced EMT. In addition, both non-Smad signaling activated by TGF-beta and cross-talk with other signaling pathways play important roles in induction of EMT. Of these, Ras signaling synergizes with TGF-beta-Smad signaling, and plays an important role in the induction of EMT. TGF-beta inhibitors prevent invasion and metastasis of advanced cancer through multiple mechanisms, including inhibition of EMT. The discovery of molecules that inhibit TGF-beta-induced EMT but not TGF-beta-induced growth arrest may be an ideal strategy for treatment of invasion and metastasis of cancer.
Figures

Similar articles
-
Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta.Mol Biol Cell. 2007 Sep;18(9):3533-44. doi: 10.1091/mbc.e07-03-0249. Epub 2007 Jul 5. Mol Biol Cell. 2007. PMID: 17615296 Free PMC article.
-
Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways.J Cell Biochem. 2005 Aug 1;95(5):918-31. doi: 10.1002/jcb.20458. J Cell Biochem. 2005. PMID: 15861394
-
TGF-beta-induced epithelial to mesenchymal transition.Cell Res. 2009 Feb;19(2):156-72. doi: 10.1038/cr.2009.5. Cell Res. 2009. PMID: 19153598 Free PMC article. Review.
-
[Aberrant Activation Mechanism of TGF-β Signaling in Epithelial-mesenchymal Transition].Yakugaku Zasshi. 2021;141(11):1229-1234. doi: 10.1248/yakushi.21-00143. Yakugaku Zasshi. 2021. PMID: 34719542 Review. Japanese.
-
TGF-β signaling and epithelial-mesenchymal transition in cancer progression.Curr Opin Oncol. 2013 Jan;25(1):76-84. doi: 10.1097/CCO.0b013e32835b6371. Curr Opin Oncol. 2013. PMID: 23197193 Review.
Cited by
-
Tumor-derived systems as novel biomedical tools-turning the enemy into an ally.Biomater Res. 2023 Nov 9;27(1):113. doi: 10.1186/s40824-023-00445-z. Biomater Res. 2023. PMID: 37946275 Free PMC article. Review.
-
Mechanism of DAPK1 for Regulating Cancer Stem Cells in Thyroid Cancer.Curr Issues Mol Biol. 2024 Jul 5;46(7):7086-7096. doi: 10.3390/cimb46070422. Curr Issues Mol Biol. 2024. PMID: 39057063 Free PMC article. Review.
-
The factor VII-activating protease (FSAP) enhances the activity of bone morphogenetic protein-2 (BMP-2).J Biol Chem. 2013 Mar 8;288(10):7193-203. doi: 10.1074/jbc.M112.433029. Epub 2013 Jan 22. J Biol Chem. 2013. PMID: 23341458 Free PMC article.
-
Association of the TGFβ gene family with microenvironmental features of gastric cancer and prediction of response to immunotherapy.Front Oncol. 2022 Sep 2;12:920599. doi: 10.3389/fonc.2022.920599. eCollection 2022. Front Oncol. 2022. PMID: 36119489 Free PMC article.
-
Identification of integrin α3 as a molecular marker of cells undergoing epithelial-mesenchymal transition and of cancer cells with aggressive phenotypes.Cancer Sci. 2013 Sep;104(9):1189-97. doi: 10.1111/cas.12220. Epub 2013 Jul 20. Cancer Sci. 2013. PMID: 23786209 Free PMC article.
References
-
- Schilling, S.H., Hjelmeland, A.B., Rich, J.N. and Wang, X.F. (2008) TGF-β: A multipotential cytokine. InThe TGF-β Family (eds. Derynck, R. and Miyazono, K.). Cold Spring Harbor Laboratory Press, New York, pp. 45–78
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

