Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling
- PMID: 19828810
- PMCID: PMC2782445
- DOI: 10.1523/JNEUROSCI.1064-09.2009
Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling
Abstract
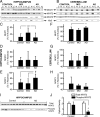
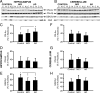
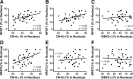
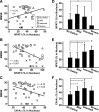
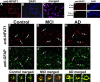
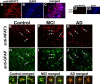
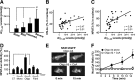
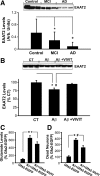
Upon activation by calcineurin, the nuclear factor of activated T-cells (NFAT) translocates to the nucleus and guides the transcription of numerous molecules involved in inflammation and Ca(2+) dysregulation, both of which are prominent features of Alzheimer's disease (AD). However, NFAT signaling in AD remains relatively uninvestigated. Using isolated cytosolic and nuclear fractions prepared from rapid-autopsy postmortem human brain tissue, we show that NFATs 1 and 3 shifted to nuclear compartments in the hippocampus at different stages of neuropathology and cognitive decline, whereas NFAT2 remained unchanged. NFAT1 exhibited greater association with isolated nuclear fractions in subjects with mild cognitive impairment (MCI), whereas NFAT3 showed a strong nuclear bias in subjects with severe dementia and AD. Similar to NFAT1, calcineurin-Aalpha also exhibited a nuclear bias in the early stages of cognitive decline. But, unlike NFAT1 and similar to NFAT3, the nuclear bias for calcineurin became more pronounced as cognition worsened. Changes in calcineurin/NFAT3 were directly correlated to soluble amyloid-beta (Abeta((1-42))) levels in postmortem hippocampus, and oligomeric Abeta, in particular, robustly stimulated NFAT activation in primary rat astrocyte cultures. Oligomeric Abeta also caused a significant reduction in excitatory amino acid transporter 2 (EAAT2) protein levels in astrocyte cultures, which was blocked by NFAT inhibition. Moreover, inhibition of astrocytic NFAT activity in mixed cultures ameliorated Abeta-dependent elevations in glutamate and neuronal death. The results suggest that NFAT signaling is selectively altered in AD and may play an important role in driving Abeta-mediated neurodegeneration.
Figures
Similar articles
-
Proteolysis of calcineurin is increased in human hippocampus during mild cognitive impairment and is stimulated by oligomeric Abeta in primary cell culture.Aging Cell. 2011 Feb;10(1):103-13. doi: 10.1111/j.1474-9726.2010.00645.x. Epub 2010 Dec 8. Aging Cell. 2011. PMID: 20969723 Free PMC article.
-
Calcineurin/NFAT Signaling in Activated Astrocytes Drives Network Hyperexcitability in Aβ-Bearing Mice.J Neurosci. 2017 Jun 21;37(25):6132-6148. doi: 10.1523/JNEUROSCI.0877-17.2017. Epub 2017 May 30. J Neurosci. 2017. PMID: 28559377 Free PMC article.
-
Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation.J Neurosci. 2010 Feb 17;30(7):2636-49. doi: 10.1523/JNEUROSCI.4456-09.2010. J Neurosci. 2010. PMID: 20164348 Free PMC article.
-
Targeting CaN/NFAT in Alzheimer's brain degeneration.Front Immunol. 2023 Nov 23;14:1281882. doi: 10.3389/fimmu.2023.1281882. eCollection 2023. Front Immunol. 2023. PMID: 38077352 Free PMC article. Review.
-
Calcineurin/NFAT signaling and innate host defence: a role for NOD1-mediated phagocytic functions.Cell Commun Signal. 2014 Jan 30;12:8. doi: 10.1186/1478-811X-12-8. Cell Commun Signal. 2014. PMID: 24479879 Free PMC article. Review.
Cited by
-
Glial Glutamine Homeostasis in Health and Disease.Neurochem Res. 2023 Apr;48(4):1100-1128. doi: 10.1007/s11064-022-03771-1. Epub 2022 Nov 2. Neurochem Res. 2023. PMID: 36322369 Review.
-
Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer's disease.J Alzheimers Dis. 2012;30(4):943-61. doi: 10.3233/JAD-2012-111661. J Alzheimers Dis. 2012. PMID: 22495349 Free PMC article.
-
The Neuroprotective Effect of the Association of Aquaporin-4/Glutamate Transporter-1 against Alzheimer's Disease.Neural Plast. 2016;2016:4626593. doi: 10.1155/2016/4626593. Epub 2016 Jan 6. Neural Plast. 2016. PMID: 27057365 Free PMC article. Review.
-
Elucidating the Interactive Roles of Glia in Alzheimer's Disease Using Established and Newly Developed Experimental Models.Front Neurol. 2018 Sep 26;9:797. doi: 10.3389/fneur.2018.00797. eCollection 2018. Front Neurol. 2018. PMID: 30319529 Free PMC article. Review.
-
Calcineurin in development and disease.Genes Dis. 2021 Mar 15;9(4):915-927. doi: 10.1016/j.gendis.2021.03.002. eCollection 2022 Jul. Genes Dis. 2021. PMID: 35685477 Free PMC article. Review.
References
-
- Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Int. 2008;52:1226–1233. - PubMed
-
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. - PubMed
-
- Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010836-14/AG/NIA NIH HHS/United States
- P50 AG005144-19/AG/NIA NIH HHS/United States
- RF1 AG027297/AG/NIA NIH HHS/United States
- P01 AG010836-13/AG/NIA NIH HHS/United States
- P01 AG010836/AG/NIA NIH HHS/United States
- K01 AG024190-04/AG/NIA NIH HHS/United States
- R01 AG027297-03/AG/NIA NIH HHS/United States
- AG024190/AG/NIA NIH HHS/United States
- AG010836/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- R01 AG027297/AG/NIA NIH HHS/United States
- K01 AG024190-05/AG/NIA NIH HHS/United States
- AG02797/AG/NIA NIH HHS/United States
- AG028383/AG/NIA NIH HHS/United States
- K01 AG024190-02/AG/NIA NIH HHS/United States
- P50 AG005144-18/AG/NIA NIH HHS/United States
- R01 AG027297-02/AG/NIA NIH HHS/United States
- P50 AG005144-17/AG/NIA NIH HHS/United States
- P01 AG010836-12/AG/NIA NIH HHS/United States
- K01 AG024190-03/AG/NIA NIH HHS/United States
- P50 AG005144-20/AG/NIA NIH HHS/United States
- K01 AG024190/AG/NIA NIH HHS/United States
- P50 AG005144/AG/NIA NIH HHS/United States
- R01 AG027297-01A1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
