Preinvasive breast cancer
- PMID: 19824828
- PMCID: PMC3918415
- DOI: 10.1146/annurev.pathol.4.110807.092306
Preinvasive breast cancer
Abstract
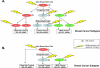
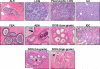
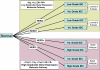
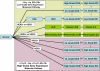
Preinvasive breast cancer accounts for approximately one-third of all newly diagnosed breast cancer cases in the United States and constitutes a spectrum of neoplastic lesions with varying degrees of differentiation and clinical behavior. High-throughput genetic, epigenetic, and gene-expression analyses have enhanced our understanding of the relationship of these early neoplastic lesions to normal breast tissue, and they strongly suggest that preinvasive breast cancer develops and evolves along two distinct molecular genetic and biological pathways that correlate with tumor grade. Although unique epigenetic and gene-expression changes are not observed in the tumor epithelial compartment during the transition from preinvasive to invasive disease, distinct molecular alterations are observed in the tumor-stromal and myoepithelial cells. This suggests that the stromal and myoepithelial microenvironment of preinvasive breast cancer actively participates in the transition from preinvasive to invasive disease. An improved understanding of the transition from preinvasive to invasive breast cancer will pave the way for novel preventative and therapeutic strategies.
Figures


Similar articles
-
Genetic relation of lobular carcinoma in situ, ductal carcinoma in situ, and associated invasive carcinoma of the breast.Mol Pathol. 2000 Jun;53(3):118-21. doi: 10.1136/mp.53.3.118. Mol Pathol. 2000. PMID: 10897329 Free PMC article.
-
Changes in tenascin-C isoform expression in invasive and preinvasive breast disease.Cancer Res. 2002 Jun 1;62(11):3289-97. Cancer Res. 2002. PMID: 12036947
-
High prevalence of preinvasive lesions adjacent to BRCA1/2-associated breast cancers.Cancer Prev Res (Phila). 2009 Feb;2(2):122-7. doi: 10.1158/1940-6207.CAPR-08-0050. Epub 2009 Jan 27. Cancer Prev Res (Phila). 2009. PMID: 19174581 Free PMC article.
-
Carcinoma in situ of the female breast. A clinico-pathological, immunohistological, and DNA ploidy study.APMIS Suppl. 2003;(108):1-67. APMIS Suppl. 2003. PMID: 12874968 Review.
-
Preinvasive lesions of the bronchus.Clin Chest Med. 2011 Dec;32(4):693-702. doi: 10.1016/j.ccm.2011.08.008. Clin Chest Med. 2011. PMID: 22054880 Review.
Cited by
-
Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia.Nature. 2014 Feb 20;506(7488):328-33. doi: 10.1038/nature13038. Epub 2014 Feb 12. Nature. 2014. PMID: 24522528 Free PMC article.
-
Sprouty4 negatively regulates ERK/MAPK signaling and the transition from in situ to invasive breast ductal carcinoma.PLoS One. 2021 May 28;16(5):e0252314. doi: 10.1371/journal.pone.0252314. eCollection 2021. PLoS One. 2021. PMID: 34048471 Free PMC article.
-
Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure.BMC Cancer. 2015 Jan 21;15:12. doi: 10.1186/s12885-015-1007-5. BMC Cancer. 2015. PMID: 25605670 Free PMC article.
-
Basal cytokeratin as a potential marker of low risk of invasion in ductal carcinoma in situ.Clinics (Sao Paulo). 2013 May;68(5):638-43. doi: 10.6061/clinics/2013(05)010. Clinics (Sao Paulo). 2013. PMID: 23778411 Free PMC article.
-
The emerging CDK4/6 inhibitor for breast cancer treatment.Mol Cell Pharmacol. 2021;13(3):9-12. Mol Cell Pharmacol. 2021. PMID: 35251463 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer (2). N Engl J Med. 1992;327:390–8. - PubMed
-
- Tavassoli FA, Devilee P. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press; 2003.
-
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–90. discussion 95-6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

