IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats
- PMID: 19822205
- PMCID: PMC2818379
- DOI: 10.1016/j.bbi.2009.10.005
IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats
Abstract
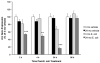
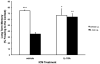
In normal aging, a peripheral immune challenge induces a sensitized and protracted neuroinflammatory response in parallel with long-term memory (LTM) impairments. Pro-inflammatory mediators of neuroinflammation impair LTM, synaptic plasticity and LTP. The immediate early gene Arc is considered a critical protein regulating LTM and synaptic plasticity. The present investigation examined whether (1) a peripheral Escherichia coli infection suppresses hippocampal Arc expression, and (2) central pro-inflammatory cytokines (IL-1beta and IL-6) mediate the effects of peripheral E. coli infection on Arc and LTM. In 24 months F344xBN F1 rats, E. coli infection suppressed basal Arc gene expression as well as contextual fear conditioning-induced Arc expression. E. coli treatment failed to alter either basal or conditioning-induced c-Fos expression. At 24h post-infection, intra-cisterna magna (ICM) treatment with the anti-inflammatory cytokine IL-1RA blocked the E. coli-induced suppression of hippocampal Arc and increases in IL-6 protein. At 4-day post-infection, IL-1RA blocked the E. coli-induced LTM impairments and increases in IL-6 protein. The present results suggest that central pro-inflammatory cytokines play a salient role in the suppression of Arc and impairments of LTM by a peripheral immune challenge in older animals.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
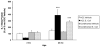
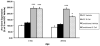
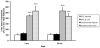

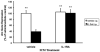
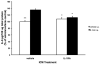
Similar articles
-
Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats.J Neurosci. 2012 Oct 17;32(42):14641-8. doi: 10.1523/JNEUROSCI.2173-12.2012. J Neurosci. 2012. PMID: 23077050 Free PMC article.
-
High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism.Brain Behav Immun. 2014 Nov;42:22-32. doi: 10.1016/j.bbi.2014.06.017. Epub 2014 Jul 3. Brain Behav Immun. 2014. PMID: 24998196 Free PMC article.
-
Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection.Brain Behav Immun. 2009 Jan;23(1):46-54. doi: 10.1016/j.bbi.2008.07.002. Epub 2008 Jul 11. Brain Behav Immun. 2009. PMID: 18664380 Free PMC article.
-
The immediate early gene arc/arg3.1: regulation, mechanisms, and function.J Neurosci. 2008 Nov 12;28(46):11760-7. doi: 10.1523/JNEUROSCI.3864-08.2008. J Neurosci. 2008. PMID: 19005037 Free PMC article. Review.
-
Transcriptional and post-translational regulation of Arc in synaptic plasticity.Semin Cell Dev Biol. 2018 May;77:3-9. doi: 10.1016/j.semcdb.2017.09.007. Epub 2017 Sep 7. Semin Cell Dev Biol. 2018. PMID: 28890422 Review.
Cited by
-
Neural Plasticity in Multiple Sclerosis: The Functional and Molecular Background.Neural Plast. 2015;2015:307175. doi: 10.1155/2015/307175. Epub 2015 Jul 2. Neural Plast. 2015. PMID: 26229689 Free PMC article. Review.
-
Cognitive and behavioral consequences of impaired immunoregulation in aging.J Neuroimmune Pharmacol. 2012 Mar;7(1):7-23. doi: 10.1007/s11481-011-9313-4. Epub 2011 Sep 20. J Neuroimmune Pharmacol. 2012. PMID: 21932047 Review.
-
Aging and an Immune Challenge Interact to Produce Prolonged, but Not Permanent, Reductions in Hippocampal L-LTP and mBDNF in a Rodent Model with Features of Delirium.eNeuro. 2018 Jun 4;5(3):ENEURO.0009-18.2018. doi: 10.1523/ENEURO.0009-18.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29911174 Free PMC article.
-
TNFα and IL-1β but not IL-18 Suppresses Hippocampal Long-Term Potentiation Directly at the Synapse.Neurochem Res. 2019 Jan;44(1):49-60. doi: 10.1007/s11064-018-2517-8. Epub 2018 Apr 4. Neurochem Res. 2019. PMID: 29619614 Free PMC article.
-
Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition.Horm Behav. 2012 Aug;62(3):219-27. doi: 10.1016/j.yhbeh.2012.02.010. Epub 2012 Feb 18. Horm Behav. 2012. PMID: 22370243 Free PMC article. Review.
References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

