Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway
- PMID: 19812319
- PMCID: PMC2787412
- DOI: 10.1523/JNEUROSCI.2939-09.2009
Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway
Abstract
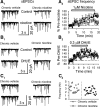
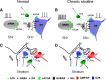
These electrophysiological experiments, in slices and intact animals, study the effects of in vivo chronic exposure to nicotine on functional alpha4beta2* nAChRs in the nigrostriatal dopaminergic (DA) pathway. Recordings were made in wild-type and alpha4 nicotinic acetylcholine receptor (nAChR) subunit knock-out mice. Chronic nicotine enhanced methyllycaconitine citrate hydrate-resistant, dihydro-beta-erythroidine hydrobromide-sensitive nicotinic currents elicited by 3-1000 mum ACh in GABAergic neurons of the substantia nigra pars reticulata (SNr), but not in DA neurons of the substantia nigra pars compacta (SNc). This enhancement leads to higher firing rates of SNr GABAergic neurons and consequently to increased GABAergic inhibition of the SNc DA neurons. In the dorsal striatum, functional alpha4* nAChRs were not found on the neuronal somata; however, nicotine acts via alpha4beta2* nAChRs in the DA terminals to modulate glutamate release onto the medium spiny neurons. Chronic nicotine also increased the number and/or function of these alpha4beta2* nAChRs. These data suggest that in nigrostriatal DA pathway, chronic nicotine enhancement of alpha4beta2* nAChRs displays selectivity in cell type and in nAChR subtype as well as in cellular compartment. These selective events augment inhibition of SNc DA neurons by SNr GABAergic neurons and also temper the release of glutamate in the dorsal striatum. The effects may reduce the risk of excitotoxicity in SNc DA neurons and may also counteract the increased effectiveness of corticostriatal glutamatergic inputs during degeneration of the DA system. These processes may contribute to the inverse correlation between tobacco use and Parkinson's disease.
Figures






Similar articles
-
A role for α4(non-α6)* nicotinic acetylcholine receptors in motor behavior.Neuropharmacology. 2013 Oct;73:19-30. doi: 10.1016/j.neuropharm.2013.05.001. Epub 2013 May 17. Neuropharmacology. 2013. PMID: 23688922 Free PMC article.
-
Iptakalim inhibits nicotinic acetylcholine receptor-mediated currents in dopamine neurons acutely dissociated from rat substantia nigra pars compacta.Neurosci Lett. 2006 Jul 31;403(1-2):57-62. doi: 10.1016/j.neulet.2006.04.060. Epub 2006 May 26. Neurosci Lett. 2006. PMID: 16730119
-
Nicotinic receptor subtype-selective circuit patterns in the subthalamic nucleus.J Neurosci. 2015 Mar 4;35(9):3734-46. doi: 10.1523/JNEUROSCI.3528-14.2015. J Neurosci. 2015. PMID: 25740504 Free PMC article.
-
Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity.Neuroscience. 2014 Dec 12;282:86-100. doi: 10.1016/j.neuroscience.2014.05.040. Epub 2014 Jun 2. Neuroscience. 2014. PMID: 24881574 Review.
-
Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: implications for drug development.Neurosci Biobehav Rev. 2007;31(3):287-314. doi: 10.1016/j.neubiorev.2006.09.008. Epub 2006 Dec 4. Neurosci Biobehav Rev. 2007. PMID: 17141870 Review.
Cited by
-
Upregulation of nAChRs and Changes in Excitability on VTA Dopamine and GABA Neurons Correlates to Changes in Nicotine-Reward-Related Behavior.eNeuro. 2020 Oct 15;7(5):ENEURO.0189-20.2020. doi: 10.1523/ENEURO.0189-20.2020. Print 2020 Sep/Oct. eNeuro. 2020. PMID: 32988984 Free PMC article.
-
86Rb+ efflux mediated by alpha4beta2*-nicotinic acetylcholine receptors with high and low-sensitivity to stimulation by acetylcholine display similar agonist-induced desensitization.Biochem Pharmacol. 2010 Oct 15;80(8):1238-51. doi: 10.1016/j.bcp.2010.06.040. Epub 2010 Jun 30. Biochem Pharmacol. 2010. PMID: 20599770 Free PMC article.
-
Dopaminergic modulation of striatal neurons, circuits, and assemblies.Neuroscience. 2011 Dec 15;198:3-18. doi: 10.1016/j.neuroscience.2011.08.051. Epub 2011 Aug 27. Neuroscience. 2011. PMID: 21906660 Free PMC article. Review.
-
Short-term auricular electrical stimulation rapidly elevated cortical blood flow and promoted the expression of nicotinic acetylcholine receptor α4 in the 2 vessel occlusion rats model.J Biomed Sci. 2019 May 11;26(1):36. doi: 10.1186/s12929-019-0526-9. J Biomed Sci. 2019. PMID: 31078140 Free PMC article.
-
Gene editing vectors for studying nicotinic acetylcholine receptors in cholinergic transmission.Eur J Neurosci. 2019 Aug;50(3):2224-2238. doi: 10.1111/ejn.13957. Epub 2018 Jul 25. Eur J Neurosci. 2019. PMID: 29779223 Free PMC article.
References
-
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. - PubMed
-
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. - PubMed
-
- Bonuccelli U, Del Dotto P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology. 2006;67:S30–S38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
