Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma
- PMID: 19809404
- PMCID: PMC2839313
- DOI: 10.1038/mt.2009.231
Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma
Abstract
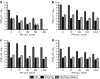
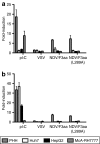
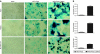
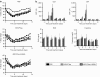
Newcastle disease virus (NDV) is an intrinsically tumor-specific virus, which is currently under investigation as a clinical oncolytic agent. Several clinical trials have reported NDV to be a safe and effective agent for cancer therapy; however, there remains a clear need for improvement in therapeutic outcome. The endogenous NDV fusion (F) protein directs membrane fusion, which is required for virus entry and cell-cell fusion. Here, we report a novel NDV vector harboring an L289A mutation within the F gene, which resulted in enhanced fusion and cytotoxicity of hepatocellular carcinoma (HCC) cells in vitro, as compared with the rNDV/F3aa control virus. In vivo administration of the recombinant vector, termed rNDV/F3aa(L289A), via hepatic arterial infusion in immune-competent Buffalo rats bearing multifocal, orthotopic liver tumors resulted in tumor-specific syncytia formation and necrosis, with no evidence of toxicity to the neighboring hepatic parenchyma. Furthermore, the improved oncolysis conferred by the L289A mutation translated to significantly prolonged survival compared with control NDV. Taken together, rNDV/F(L289A) represents a safe, yet more effective vector than wild-type NDV for the treatment of HCC, making it an ideal candidate for clinical application in HCC patients.
Figures


Similar articles
-
A Novel Chimeric Oncolytic Virus Vector for Improved Safety and Efficacy as a Platform for the Treatment of Hepatocellular Carcinoma.J Virol. 2018 Nov 12;92(23):e01386-18. doi: 10.1128/JVI.01386-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232179 Free PMC article.
-
The potential of recombinant vesicular stomatitis virus-mediated virotherapy against metastatic colon cancer.Int J Mol Med. 2013 Feb;31(2):299-306. doi: 10.3892/ijmm.2012.1205. Epub 2012 Dec 6. Int J Mol Med. 2013. PMID: 23232984
-
Oncolytic Newcastle disease virus expressing chimeric antibody enhanced anti-tumor efficacy in orthotopic hepatoma-bearing mice.J Exp Clin Cancer Res. 2015 Dec 21;34:153. doi: 10.1186/s13046-015-0271-1. J Exp Clin Cancer Res. 2015. PMID: 26689432 Free PMC article.
-
[Expressing foreign genes by Newcastle disease virus for cancer therapy].Mol Biol (Mosk). 2015 Mar-Apr;49(2):195-204. doi: 10.7868/s0026898415020020. Mol Biol (Mosk). 2015. PMID: 26065249 Review. Russian.
-
Oncolytic therapy and gene therapy for cancer: recent advances in antitumor effects of Newcastle disease virus.Discov Med. 2020 Jul-Aug;30(159):39-48. Discov Med. 2020. PMID: 33357361 Review.
Cited by
-
Oncolytic virus-based hepatocellular carcinoma treatment: Current status, intravenous delivery strategies, and emerging combination therapeutic solutions.Asian J Pharm Sci. 2023 Jan;18(1):100771. doi: 10.1016/j.ajps.2022.100771. Epub 2022 Dec 29. Asian J Pharm Sci. 2023. PMID: 36896445 Free PMC article. Review.
-
The Viral Knock: Ameliorating Cancer Treatment with Oncolytic Newcastle Disease Virus.Life (Basel). 2023 Jul 26;13(8):1626. doi: 10.3390/life13081626. Life (Basel). 2023. PMID: 37629483 Free PMC article. Review.
-
Replication and Oncolytic Activity of an Avian Orthoreovirus in Human Hepatocellular Carcinoma Cells.Viruses. 2017 Apr 24;9(4):90. doi: 10.3390/v9040090. Viruses. 2017. PMID: 28441762 Free PMC article.
-
Newcastle disease virus: current status and our understanding.Virus Res. 2014 May 12;184:71-81. doi: 10.1016/j.virusres.2014.02.016. Epub 2014 Mar 1. Virus Res. 2014. PMID: 24589707 Free PMC article. Review.
-
Oncolytic virotherapy in upper gastrointestinal tract cancers.Oncolytic Virother. 2018 Mar 23;7:13-24. doi: 10.2147/OV.S161397. eCollection 2017. Oncolytic Virother. 2018. PMID: 29616200 Free PMC article. Review.
References
-
- Parkin DM, Bray F, Ferlay J., and , Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. - PubMed
-
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. - PubMed
-
- Dyer Z, Peltekian K., and , van Zanten SV. Review article: the changing epidemiology of hepatocellular carcinoma in Canada. Aliment Pharmacol Ther. 2005;22:17–22. - PubMed
-
- El-Serag HB, Davila JA, Petersen NJ., and , McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. - PubMed
-
- Deuffic S, Poynard T, Buffat L., and , Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351:214–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

