Characterization of cytoplasmic caspase-2 activation by induced proximity
- PMID: 19782032
- PMCID: PMC2755603
- DOI: 10.1016/j.molcel.2009.07.023
Characterization of cytoplasmic caspase-2 activation by induced proximity
Abstract
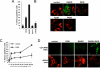
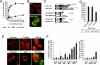
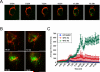
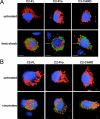
Caspase-2 is an initiator caspase activated in response to heat shock and other stressors that induce apoptosis. Activation of caspase-2 requires induced proximity resulting after recruitment to caspase-2 activation complexes such as the PIDDosome. We have adapted bimolecular fluorescence complementation (BiFC) to measure caspase-2 induced proximity in real time in single cells. Nonfluorescent fragments of the fluorescent protein Venus that can associate to reform the fluorescent complex were fused to caspase-2, allowing visualization and kinetic measurements of caspase-2 induced proximity after heat shock and other stresses. This revealed that the caspase-2 activation platform occurred in the cytosol and not in the nucleus in response to heat shock, DNA damage, cytoskeletal disruption, and other treatments. Activation, as measured by this approach, in response to heat shock was RAIDD dependent and upstream of mitochondrial outer-membrane permeabilization. Furthermore, we identify Hsp90alpha as a key negative regulator of heat shock-induced caspase-2 activation.
Figures
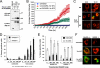
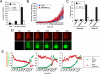

Comment in
-
A cut above the other caspases.Mol Cell. 2009 Sep 24;35(6):733-4. doi: 10.1016/j.molcel.2009.09.001. Mol Cell. 2009. PMID: 19782021
Similar articles
-
A cut above the other caspases.Mol Cell. 2009 Sep 24;35(6):733-4. doi: 10.1016/j.molcel.2009.09.001. Mol Cell. 2009. PMID: 19782021
-
NPM1 directs PIDDosome-dependent caspase-2 activation in the nucleolus.J Cell Biol. 2017 Jun 5;216(6):1795-1810. doi: 10.1083/jcb.201608095. Epub 2017 Apr 21. J Cell Biol. 2017. PMID: 28432080 Free PMC article.
-
Heat shock induces apoptosis independently of any known initiator caspase-activating complex.J Biol Chem. 2006 Jun 23;281(25):16991-17000. doi: 10.1074/jbc.M512754200. Epub 2006 Apr 17. J Biol Chem. 2006. PMID: 16618700
-
Caspase-2: the orphan caspase.Cell Death Differ. 2012 Jan;19(1):51-7. doi: 10.1038/cdd.2011.157. Epub 2011 Nov 11. Cell Death Differ. 2012. PMID: 22075987 Free PMC article. Review.
-
Total recall: the role of PIDDosome components in neurodegeneration.Trends Mol Med. 2023 Dec;29(12):996-1013. doi: 10.1016/j.molmed.2023.08.008. Epub 2023 Sep 14. Trends Mol Med. 2023. PMID: 37716905 Review.
Cited by
-
A noncanonical IRAK4-IRAK1 pathway counters DNA damage-induced apoptosis independently of TLR/IL-1R signaling.Sci Signal. 2023 Dec 19;16(816):eadh3449. doi: 10.1126/scisignal.adh3449. Epub 2023 Dec 19. Sci Signal. 2023. PMID: 38113335 Free PMC article.
-
Metacaspase Yca1 is required for clearance of insoluble protein aggregates.Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13348-53. doi: 10.1073/pnas.1006610107. Epub 2010 Jul 12. Proc Natl Acad Sci U S A. 2010. PMID: 20624963 Free PMC article.
-
Cell death controlling complexes and their potential therapeutic role.Cell Mol Life Sci. 2015 Feb;72(3):505-517. doi: 10.1007/s00018-014-1757-2. Epub 2014 Oct 17. Cell Mol Life Sci. 2015. PMID: 25323133 Free PMC article. Review.
-
Cell Death Signaling.Cold Spring Harb Perspect Biol. 2015 Dec 1;7(12):a006080. doi: 10.1101/cshperspect.a006080. Cold Spring Harb Perspect Biol. 2015. PMID: 26626938 Free PMC article. Review.
-
Analysis of the minimal specificity of caspase-2 and identification of Ac-VDTTD-AFC as a caspase-2-selective peptide substrate.Biosci Rep. 2014 Apr 1;34(2):e00100. doi: 10.1042/BSR20140025. Print 2014 Apr 1. Biosci Rep. 2014. PMID: 27919034 Free PMC article.
References
-
- Arlander S, Eapen A, Vroman B, McDonald R, Toft D, Karnitz L. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278:52572–52577. - PubMed
-
- Baliga BC, Colussi PA, Read SH, Dias MM, Jans DA, Kumar S. Role of prodomain in importin-mediated nuclear localization and activation of caspase-2. J Biol Chem. 2003;278:4899–4905. - PubMed
-
- Baliga BC, Read SH, Kumar S. The biochemical mechanism of caspase-2 activation. Cell Death Differ. 2004;11:1234–1241. - PubMed
-
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

