The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels
- PMID: 19778904
- PMCID: PMC2781673
- DOI: 10.1074/jbc.M109.019687
The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels
Abstract
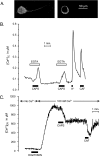
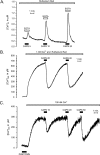
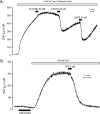
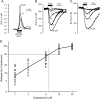
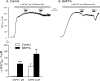
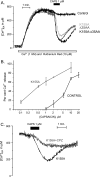
Transient receptor potential vanilloid type 1 (TRPV1) is a plasma membrane Ca(2+) channel involved in transduction of painful stimuli. Dorsal root ganglion (DRG) neurons express ectopic but functional TRPV1 channels in the endoplasmic reticulum (ER) (TRPV1(ER)). We have studied the properties of TRPV1(ER) in DRG neurons and HEK293T cells expressing TRPV1. Activation of TRPV1(ER) with capsaicin or other vanilloids produced an increase of cytosolic Ca(2+) due to Ca(2+) release from the ER. The decrease of [Ca(2+)](ER) was directly revealed by an ER-targeted aequorin Ca(2+) probe, expressed in DRG neurons using a herpes amplicon virus. The sensitivity of TRPV1(ER) to capsaicin was smaller than the sensitivity of the plasma membrane TRPV1 channels. The low affinity of TRPV1(ER) was not related to protein kinase A- or C-mediated phosphorylations, but it was due to inactivation by cytosolic Ca(2+) because the sensitivity to capsaicin was increased by loading the cells with the Ca(2+) chelator BAPTA. Decreasing [Ca(2+)](ER) did not affect the sensitivity of TRPV1(ER) to capsaicin. Disruption of the TRPV1 calmodulin-binding domains at either the C terminus (Delta35AA) or the N terminus (K155A) increased 10-fold the affinity of TRPV1(ER) for capsaicin, suggesting that calmodulin is involved in the inactivation. The lack of TRPV1 sensitizers, such as phosphatidylinositol 4,5-bisphosphate, in the ER could contribute to decrease the affinity for capsaicin. The low sensitivity of TRPV1(ER) to agonists may be critical for neuron health, because otherwise Ca(2+) depletion of ER could lead to ER stress, unfolding protein response, and cell death.
Figures


Similar articles
-
Local Ca2+ signals couple activation of TRPV1 and ANO1 sensory ion channels.Sci Signal. 2020 Apr 28;13(629):eaaw7963. doi: 10.1126/scisignal.aaw7963. Sci Signal. 2020. PMID: 32345727 Free PMC article.
-
Mechanism of capsaicin receptor TRPV1-mediated toxicity in pain-sensing neurons focusing on the effects of Na(+)/Ca(2+) fluxes and the Ca(2+)-binding protein calretinin.Biochim Biophys Acta. 2013 Jul;1833(7):1680-91. doi: 10.1016/j.bbamcr.2012.08.018. Epub 2012 Sep 5. Biochim Biophys Acta. 2013. PMID: 22982061
-
Hypericum perforatum Attenuates Spinal Cord Injury-Induced Oxidative Stress and Apoptosis in the Dorsal Root Ganglion of Rats: Involvement of TRPM2 and TRPV1 Channels.Mol Neurobiol. 2016 Aug;53(6):3540-3551. doi: 10.1007/s12035-015-9292-1. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26099309
-
Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells.Int J Mol Sci. 2020 Jun 11;21(11):4177. doi: 10.3390/ijms21114177. Int J Mol Sci. 2020. PMID: 32545311 Free PMC article. Review.
-
Phosphoinositide regulation of TRPV1 revisited.Pflugers Arch. 2015 Sep;467(9):1851-69. doi: 10.1007/s00424-015-1695-3. Epub 2015 Mar 11. Pflugers Arch. 2015. PMID: 25754030 Free PMC article. Review.
Cited by
-
Capsaicin Blocks the Hyperpolarization-Activated Inward Currents via TRPV1 in the Rat Dorsal Root Ganglion Neurons.Exp Neurobiol. 2012 Jun;21(2):75-82. doi: 10.5607/en.2012.21.2.75. Epub 2012 Jun 12. Exp Neurobiol. 2012. PMID: 22792028 Free PMC article.
-
Transient receptor potential vanilloid type 1 channel (TRPV1) immunolocalization in the murine enteric nervous system is affected by the targeted C-terminal epitope of the applied antibody.J Histochem Cytochem. 2013 Jun;61(6):421-32. doi: 10.1369/0022155413484764. Epub 2013 Mar 12. J Histochem Cytochem. 2013. PMID: 23482327 Free PMC article.
-
Intact microtubules preserve transient receptor potential vanilloid 1 (TRPV1) functionality through receptor binding.J Biol Chem. 2012 Mar 2;287(10):7803-11. doi: 10.1074/jbc.M111.332296. Epub 2012 Jan 17. J Biol Chem. 2012. PMID: 22262838 Free PMC article.
-
Local Ca2+ signals couple activation of TRPV1 and ANO1 sensory ion channels.Sci Signal. 2020 Apr 28;13(629):eaaw7963. doi: 10.1126/scisignal.aaw7963. Sci Signal. 2020. PMID: 32345727 Free PMC article.
-
Cannabinoids Modulate Neuronal Activity and Cancer by CB1 and CB2 Receptor-Independent Mechanisms.Front Pharmacol. 2017 Oct 10;8:720. doi: 10.3389/fphar.2017.00720. eCollection 2017. Front Pharmacol. 2017. PMID: 29066974 Free PMC article. Review.
References
-
- Caterina M. J., Julius D. (2001) Annu. Rev. Neurosci. 24, 487–517 - PubMed
-
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) Nature 389, 816–824 - PubMed
-
- Dray A., Forbes C. A., Burgess G. M. (1990) Neurosci. Lett. 110, 52–59 - PubMed
-
- Zhu M. X. (2005) Pflugers Arch. 451, 105–115 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

