Group I mGluR activation enhances Ca(2+)-dependent nonselective cation currents and rhythmic bursting in main olfactory bulb external tufted cells
- PMID: 19776280
- PMCID: PMC3837548
- DOI: 10.1523/JNEUROSCI.0206-09.2009
Group I mGluR activation enhances Ca(2+)-dependent nonselective cation currents and rhythmic bursting in main olfactory bulb external tufted cells
Abstract
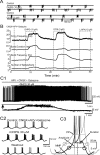
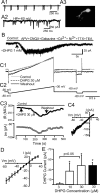
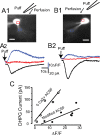
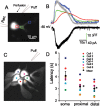
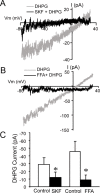
In the main olfactory bulb, activation of group I metabotropic glutamate receptors (mGluRs) by olfactory nerve stimulation generates slow (2 Hz) oscillations near the basal respiratory frequency. These oscillations arise in the glomerular layer and may be generated, in part, by the intrinsic neurons, the juxtaglomerular neurons. We investigated the physiological effects of group I mGluR agonists on one population of juxtaglomerular neurons, external tufted (ET) cells, which rhythmically burst at respiratory frequencies and synchronize the intraglomerular network. Electrophysiological studies in rat main olfactory bulb slices demonstrated that the mGluR agonist 3,4-dihydroxyphenylglycine (DHPG) amplified the strength of ET cell spike bursts, principally by increasing the number of spikes per burst. Voltage-clamp and Ca(2+)-imaging studies showed that DHPG elicits a Ca(2+)-dependent nonselective cation current (I(CAN)) in the dendrites of ET cells triggered by Ca(2+) release from internal stores. The DHPG effects on bursting and membrane current were attenuated by flufenamic acid and SKF96365, agents known to antagonize I(CAN) in a variety of neurons. DHPG also elicited slow membrane current oscillations and spikelets in ET cells when synaptic transmission and intrinsic membrane channels were inoperative. These findings indicate that DHPG may passively (by increasing burst strength) or actively (by increasing conductance of gap junctions) enhance the strength of electrical synapses between ET cells. Together, these findings indicate that activation of group I mGluRs on the dendrites of ET cells play a key role in the generation of slow rhythmic oscillation in the glomerular network, which is in turn tuned to sniffing of the animal in vivo.
Figures


Similar articles
-
Activation of group I metabotropic glutamate receptors enhances persistent sodium current and rhythmic bursting in main olfactory bulb external tufted cells.J Neurophysiol. 2014 Feb;111(3):641-7. doi: 10.1152/jn.00696.2013. Epub 2013 Nov 13. J Neurophysiol. 2014. PMID: 24225539 Free PMC article.
-
Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input.J Neurosci. 2004 Feb 4;24(5):1190-9. doi: 10.1523/JNEUROSCI.4714-03.2004. J Neurosci. 2004. PMID: 14762137 Free PMC article.
-
Metabotropic glutamate receptors in the main olfactory bulb drive granule cell-mediated inhibition.J Neurophysiol. 2007 Jan;97(1):858-70. doi: 10.1152/jn.00884.2006. Epub 2006 Nov 8. J Neurophysiol. 2007. PMID: 17093122 Free PMC article.
-
Synaptic activation of T-type Ca2+ channels via mGluR activation in the primary dendrite of mitral cells.J Neurophysiol. 2010 May;103(5):2557-69. doi: 10.1152/jn.00796.2009. Epub 2010 Jan 13. J Neurophysiol. 2010. PMID: 20071628
-
Novel modes of rhythmic burst firing at cognitively-relevant frequencies in thalamocortical neurons.Brain Res. 2008 Oct 15;1235:12-20. doi: 10.1016/j.brainres.2008.06.029. Epub 2008 Jun 19. Brain Res. 2008. PMID: 18602904 Free PMC article. Review.
Cited by
-
Metabotropic glutamate receptors (mGluR5) activate transient receptor potential canonical channels to improve the regularity of the respiratory rhythm generated by the pre-Bötzinger complex in mice.Eur J Neurosci. 2012 Jun;35(11):1725-37. doi: 10.1111/j.1460-9568.2012.08091.x. Epub 2012 May 22. Eur J Neurosci. 2012. PMID: 22612431 Free PMC article.
-
Cellular and Synaptic Mechanisms That Differentiate Mitral Cells and Superficial Tufted Cells Into Parallel Output Channels in the Olfactory Bulb.Front Cell Neurosci. 2020 Dec 22;14:614377. doi: 10.3389/fncel.2020.614377. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33414707 Free PMC article.
-
Expression of transient receptor potential (TRP) channel mRNAs in the mouse olfactory bulb.Neurosci Lett. 2012 Aug 22;524(1):49-54. doi: 10.1016/j.neulet.2012.07.013. Epub 2012 Jul 20. Neurosci Lett. 2012. PMID: 22820212 Free PMC article.
-
Activation of group I metabotropic glutamate receptors modulates locomotor-related motoneuron output in mice.J Neurophysiol. 2011 May;105(5):2108-20. doi: 10.1152/jn.01037.2010. Epub 2011 Feb 23. J Neurophysiol. 2011. PMID: 21346211 Free PMC article.
-
Molars to Medicine: A Focused Review on the Pre-Clinical Investigation and Treatment of Secondary Degeneration following Spinal Cord Injury Using Dental Stem Cells.Cells. 2024 May 10;13(10):817. doi: 10.3390/cells13100817. Cells. 2024. PMID: 38786039 Free PMC article. Review.
References
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;29:83–120. - PubMed
-
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. - PubMed
-
- Buonviso N, Amat C, Litaudon P. Respiratory modulation of olfactory neurons in the rodent brain. Chem Senses. 2006;31:145–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

