In silico reconstitution of actin-based symmetry breaking and motility
- PMID: 19771152
- PMCID: PMC2738636
- DOI: 10.1371/journal.pbio.1000201
In silico reconstitution of actin-based symmetry breaking and motility
Abstract
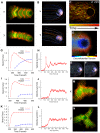
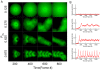
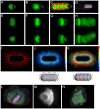
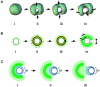
Eukaryotic cells assemble viscoelastic networks of crosslinked actin filaments to control their shape, mechanical properties, and motility. One important class of actin network is nucleated by the Arp2/3 complex and drives both membrane protrusion at the leading edge of motile cells and intracellular motility of pathogens such as Listeria monocytogenes. These networks can be reconstituted in vitro from purified components to drive the motility of spherical micron-sized beads. An Elastic Gel model has been successful in explaining how these networks break symmetry, but how they produce directed motile force has been less clear. We have combined numerical simulations with in vitro experiments to reconstitute the behavior of these motile actin networks in silico using an Accumulative Particle-Spring (APS) model that builds on the Elastic Gel model, and demonstrates simple intuitive mechanisms for both symmetry breaking and sustained motility. The APS model explains observed transitions between smooth and pulsatile motion as well as subtle variations in network architecture caused by differences in geometry and conditions. Our findings also explain sideways symmetry breaking and motility of elongated beads, and show that elastic recoil, though important for symmetry breaking and pulsatile motion, is not necessary for smooth directional motility. The APS model demonstrates how a small number of viscoelastic network parameters and construction rules suffice to recapture the complex behavior of motile actin networks. The fact that the model not only mirrors our in vitro observations, but also makes novel predictions that we confirm by experiment, suggests that the model captures much of the essence of actin-based motility in this system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
Blasting off on an actin comet tail.PLoS Biol. 2009 Sep 22;7(9):e1000195. doi: 10.1371/journal.pbio.1000195. PLoS Biol. 2009. PMID: 20076753 Free PMC article. No abstract available.
Similar articles
-
Cooperative symmetry-breaking by actin polymerization in a model for cell motility.Nat Cell Biol. 1999 Dec;1(8):493-9. doi: 10.1038/70281. Nat Cell Biol. 1999. PMID: 10587645
-
Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex.Cell. 2008 May 30;133(5):841-51. doi: 10.1016/j.cell.2008.04.011. Cell. 2008. PMID: 18510928 Free PMC article.
-
Myosin-I synergizes with Arp2/3 complex to enhance the pushing forces of branched actin networks.Sci Adv. 2024 Sep 13;10(37):eado5788. doi: 10.1126/sciadv.ado5788. Epub 2024 Sep 13. Sci Adv. 2024. PMID: 39270022 Free PMC article.
-
Listeria comet tails: the actin-based motility machinery at work.Trends Cell Biol. 2008 May;18(5):220-7. doi: 10.1016/j.tcb.2008.03.001. Epub 2008 Apr 7. Trends Cell Biol. 2008. PMID: 18396046 Review.
-
Actin-based motility as a self-organized system: mechanism and reconstitution in vitro.C R Biol. 2003 Feb;326(2):161-70. doi: 10.1016/s1631-0691(03)00067-2. C R Biol. 2003. PMID: 12754935 Review.
Cited by
-
Purification of native Arp2/3 complex from bovine thymus.Methods Mol Biol. 2013;1046:231-50. doi: 10.1007/978-1-62703-538-5_14. Methods Mol Biol. 2013. PMID: 23868592 Free PMC article.
-
Quantitative regulation of the dynamic steady state of actin networks.Elife. 2019 Mar 14;8:e42413. doi: 10.7554/eLife.42413. Elife. 2019. PMID: 30869077 Free PMC article.
-
Actin dynamics: from nanoscale to microscale.Annu Rev Biophys. 2010;39:91-110. doi: 10.1146/annurev.biophys.093008.131207. Annu Rev Biophys. 2010. PMID: 20462375 Free PMC article. Review.
-
Cease-fire at the leading edge: new perspectives on actin filament branching, debranching, and cross-linking.Cytoskeleton (Hoboken). 2011 Nov;68(11):596-602. doi: 10.1002/cm.20543. Epub 2011 Oct 28. Cytoskeleton (Hoboken). 2011. PMID: 22002930 Free PMC article. Review.
-
CPI motif interaction is necessary for capping protein function in cells.Nat Commun. 2015 Sep 28;6:8415. doi: 10.1038/ncomms9415. Nat Commun. 2015. PMID: 26412145 Free PMC article.
References
-
- Pollard T. D, Earnshaw W. C, Lippincott-Schwartz J. Cell biology. Philadelphia, PA: Saunders/Elsevier; 2008. 905
-
- Chhabra E. S, Higgs H. N. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. - PubMed
-
- Nakamura F, Osborn E, Janmey P. A, Stossel T. P. Comparison of filamin A-induced cross-linking and Arp2/3 complex-mediated branching on the mechanics of actin filaments. J Biol Chem. 2002;277:9148–9154. - PubMed

