Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both?
- PMID: 19763230
- PMCID: PMC2744987
- DOI: 10.2217/17460794.3.3.265
Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both?
Abstract
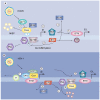
The promyelocytic leukemia (PML) protein gathers other cellular proteins, such as Daxx and Sp100, to form subnuclear structures termed PML-nuclear bodies (PML-NBs) or ND10 domains. Many infecting viral genomes localize to PML-NBs, leading to speculation that these structures may represent the most efficient subnuclear location for viral replication. Conversely, many viral proteins modify or disrupt PML-NBs, suggesting that viral replication may be more efficient in the absence of these structures. Thus, a debate remains as to whether PML-NBs inhibit or enhance viral replication. Here we review and discuss recent data indicating that for herpesviruses, PML-NB proteins inhibit viral replication in cell types where productive, lytic replication occurs, while at the same time may enhance the establishment of lifelong latent infections in other cell types.
Figures
Similar articles
-
Promyelocytic leukemia (PML) nuclear bodies (NBs) induce latent/quiescent HSV-1 genomes chromatinization through a PML NB/Histone H3.3/H3.3 Chaperone Axis.PLoS Pathog. 2018 Sep 20;14(9):e1007313. doi: 10.1371/journal.ppat.1007313. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30235352 Free PMC article.
-
An Adenovirus DNA Replication Factor, but Not Incoming Genome Complexes, Targets PML Nuclear Bodies.J Virol. 2015 Nov 25;90(3):1657-67. doi: 10.1128/JVI.02545-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26608315 Free PMC article.
-
Lytic but not latent replication of epstein-barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins.J Virol. 2000 Dec;74(24):11800-10. doi: 10.1128/jvi.74.24.11800-11810.2000. J Virol. 2000. PMID: 11090180 Free PMC article.
-
The Role of Promyelocytic Leukemia Nuclear Bodies During HPV Infection.Front Cell Infect Microbiol. 2020 Feb 19;10:35. doi: 10.3389/fcimb.2020.00035. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32154186 Free PMC article. Review.
-
Viruses as hijackers of PML nuclear bodies.Arch Immunol Ther Exp (Warsz). 2003;51(5):295-300. Arch Immunol Ther Exp (Warsz). 2003. PMID: 14626429 Review.
Cited by
-
Elongin B-mediated epigenetic alteration of viral chromatin correlates with efficient human cytomegalovirus gene expression and replication.mBio. 2011 Mar 29;2(2):e00023-11. doi: 10.1128/mBio.00023-11. Print 2011. mBio. 2011. PMID: 21447700 Free PMC article.
-
Cyclin-dependent kinase-like function is shared by the beta- and gamma- subset of the conserved herpesvirus protein kinases.PLoS Pathog. 2010 Sep 9;6(9):e1001092. doi: 10.1371/journal.ppat.1001092. PLoS Pathog. 2010. PMID: 20838604 Free PMC article.
-
Pleiotropic functions of EAPII/TTRAP/TDP2: cancer development, chemoresistance and beyond.Cell Cycle. 2011 Oct 1;10(19):3274-83. doi: 10.4161/cc.10.19.17763. Epub 2011 Oct 1. Cell Cycle. 2011. PMID: 21926483 Free PMC article. Review.
-
Immediate-Early (IE) gene regulation of cytomegalovirus: IE1- and pp71-mediated viral strategies against cellular defenses.Virol Sin. 2014 Dec;29(6):343-52. doi: 10.1007/s12250-014-3532-9. Epub 2014 Nov 25. Virol Sin. 2014. PMID: 25501994 Free PMC article. Review.
-
Cellular promyelocytic leukemia protein is an important dengue virus restriction factor.PLoS One. 2015 May 11;10(5):e0125690. doi: 10.1371/journal.pone.0125690. eCollection 2015. PLoS One. 2015. PMID: 25962098 Free PMC article.
References
-
-
Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 2004;5:1109–1115.
Review defining intrinsic immunity .
-
-
-
Saffert RT, Kalejta RF. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 2006;80:3863–3871.First demonstration of the ability of a promyelocytic leukemia-nuclear body (PML-NB) protein to mediate intrinsic immune defense against a DNA virus.
-
-
-
Tavalai N, Papior P, Rechter S, Stamminger T. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J. Virol. 2008;82:126–137.Evidence that individual PML-NB proteins can have independent effects on herpesviral gene expression.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
