Evolution of the neocortex: a perspective from developmental biology
- PMID: 19763105
- PMCID: PMC2913577
- DOI: 10.1038/nrn2719
Evolution of the neocortex: a perspective from developmental biology
Abstract
The enlargement and species-specific elaboration of the cerebral neocortex during evolution holds the secret to the mental abilities of humans; however, the genetic origin and cellular mechanisms that generated the distinct evolutionary advancements are not well understood. This article describes how novelties that make us human may have been introduced during evolution, based on findings in the embryonic cerebral cortex in different mammalian species. The data on the differences in gene expression, new molecular pathways and novel cellular interactions that have led to these evolutionary advances may also provide insight into the pathogenesis and therapies for human-specific neuropsychiatric disorders.
Figures

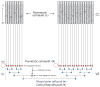

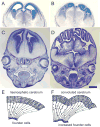

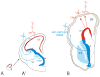

Similar articles
-
Cajal-Retzius cells and the development of the neocortex.Trends Neurosci. 1998 Feb;21(2):64-71. doi: 10.1016/s0166-2236(97)01164-8. Trends Neurosci. 1998. PMID: 9498301 Review.
-
Neocortical neurogenesis is not really "neo": a new evolutionary model derived from a comparative study of chick pallial development.Dev Growth Differ. 2013 Jan;55(1):173-87. doi: 10.1111/dgd.12020. Epub 2012 Dec 12. Dev Growth Differ. 2013. PMID: 23230908 Review.
-
Origin and evolution of developmental enhancers in the mammalian neocortex.Proc Natl Acad Sci U S A. 2016 May 10;113(19):E2617-26. doi: 10.1073/pnas.1603718113. Epub 2016 Apr 25. Proc Natl Acad Sci U S A. 2016. PMID: 27114548 Free PMC article.
-
How unique is the human neocortex?Development. 2014 Jan;141(1):11-6. doi: 10.1242/dev.101279. Development. 2014. PMID: 24346696
-
Human neocortical development: the importance of embryonic and early fetal events.Neuroscientist. 2001 Aug;7(4):303-14. doi: 10.1177/107385840100700407. Neuroscientist. 2001. PMID: 11488396 Review.
Cited by
-
General hallmarks of microRNAs in brain evolution and development.RNA Biol. 2015;12(7):701-8. doi: 10.1080/15476286.2015.1048954. RNA Biol. 2015. PMID: 26000728 Free PMC article. Review.
-
Large-scale genomics unveil polygenic architecture of human cortical surface area.Nat Commun. 2015 Jul 20;6:7549. doi: 10.1038/ncomms8549. Nat Commun. 2015. PMID: 26189703 Free PMC article.
-
Cross-site reproducibility of human cortical organoids reveals consistent cell type composition and architecture.Stem Cell Reports. 2024 Sep 10;19(9):1351-1367. doi: 10.1016/j.stemcr.2024.07.008. Epub 2024 Aug 22. Stem Cell Reports. 2024. PMID: 39178845 Free PMC article.
-
Mutant Huntingtin Drives Development of an Advantageous Brain Early in Life: Evidence in Support of Antagonistic Pleiotropy.Ann Neurol. 2024 Nov;96(5):1006-1019. doi: 10.1002/ana.27046. Epub 2024 Aug 8. Ann Neurol. 2024. PMID: 39115048
-
Lost highway(s): barriers to postnatal cortical neurogenesis and implications for brain repair.Front Cell Neurosci. 2015 Jun 16;9:216. doi: 10.3389/fncel.2015.00216. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26136658 Free PMC article. Review.
References
-
- Striedter GF. Principles of Brain Evolution. Sinauer; Sunderland, MA: 2005.
-
- Northcutt RG. Evolution of the telencephalon in non-mammals. Ann Rev Neurosci. 1981;4:301–350. - PubMed
-
- Murphy WJ, Pevzner PA, O’Brian SJ. Mammalian phylogenomic comes of age. Trends Genet. 2004;20:631–9. A concise and informative review of the DNA sequencing-based time-scale of phylogenetic divergence of various mammalian species. - PubMed
-
- Preuss TM. The cognitive neuroscience of human uniqueness. In: Gazzaniga MS, editor. The Cognitive Neuroscience IV. The MIT Press; Cambridge, MA: 2009. in the press.
-
- Goffinet AM, Rakic P, editors. Mouse Brain Development. Springer-Verlag; Berlin; New York: 2000.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

