Dissecting dynamin's role in clathrin-mediated endocytosis
- PMID: 19754444
- PMCID: PMC2879887
- DOI: 10.1042/BST0371022
Dissecting dynamin's role in clathrin-mediated endocytosis
Abstract
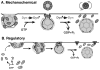
The GTPase dynamin is essential for CME (clathrin-mediated endocytosis), but its exact function and mechanism of action have been controversial. Here, we review findings that have led to the current models for dynamin function, either as a mechanochemical enzyme driving membrane fission or as a regulatory GTPase monitoring rate-limiting steps in CME. However, these models are not mutually exclusive and subsequent studies have provided evidence for both dynamin functions. Recent evidence derived from divergent in vivo and in vitro approaches suggests that dynamin plays a dual role in CME, functioning at early stages as a fidelity monitor to regulate clathrin-coated pit maturation and at later stages to directly catalyse membrane fission and clathrin-coated vesicle formation.
Figures


Similar articles
-
Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis.J Cell Biol. 2000 Sep 4;150(5):1137-48. doi: 10.1083/jcb.150.5.1137. J Cell Biol. 2000. PMID: 10974001 Free PMC article.
-
Early and nonredundant functions of dynamin isoforms in clathrin-mediated endocytosis.Mol Biol Cell. 2020 Aug 15;31(18):2035-2047. doi: 10.1091/mbc.E20-06-0363. Epub 2020 Jun 24. Mol Biol Cell. 2020. PMID: 32579424 Free PMC article.
-
Dynamin: functional design of a membrane fission catalyst.Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review.
-
Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis.Curr Biol. 1999 Mar 11;9(5):257-60. doi: 10.1016/s0960-9822(99)80114-6. Curr Biol. 1999. PMID: 10074456
-
Dynamin rings: not just for fission.Traffic. 2013 Dec;14(12):1194-9. doi: 10.1111/tra.12116. Epub 2013 Sep 19. Traffic. 2013. PMID: 23980695 Free PMC article. Review.
Cited by
-
Actin bundling by dynamin 2 and cortactin is implicated in cell migration by stabilizing filopodia in human non-small cell lung carcinoma cells.Int J Oncol. 2016 Sep;49(3):877-86. doi: 10.3892/ijo.2016.3592. Epub 2016 Jun 30. Int J Oncol. 2016. PMID: 27572123 Free PMC article.
-
G domain dimerization controls dynamin's assembly-stimulated GTPase activity.Nature. 2010 May 27;465(7297):435-40. doi: 10.1038/nature09032. Epub 2010 Apr 28. Nature. 2010. PMID: 20428113 Free PMC article.
-
Entry pathways of herpes simplex virus type 1 into human keratinocytes are dynamin- and cholesterol-dependent.PLoS One. 2011;6(10):e25464. doi: 10.1371/journal.pone.0025464. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022400 Free PMC article.
-
GSK3α phosphorylates dynamin-2 to promote GLUT4 endocytosis in muscle cells.J Cell Biol. 2023 Feb 6;222(2):e202102119. doi: 10.1083/jcb.202102119. Epub 2022 Nov 29. J Cell Biol. 2023. PMID: 36445308 Free PMC article.
-
The dynamin inhibitor dynasore inhibits bone resorption by rapidly disrupting actin rings of osteoclasts.J Bone Miner Metab. 2016 Jul;34(4):395-405. doi: 10.1007/s00774-015-0683-1. Epub 2015 Jun 11. J Bone Miner Metab. 2016. PMID: 26063501
References
-
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Yarar D, Surka MC, Leonard MC, Schmid SL. Snx9 activities are regulated by multiple phosphoinositides through both px and bar domains. Traffic. 2008;9:133–146. - PubMed
-
- Sever S, Muhlberg AB, Schmid SL. Impairment of dynamin’s GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

