The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not
- PMID: 19749171
- PMCID: PMC2763780
- DOI: 10.1124/pr.109.001263
The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not
Abstract
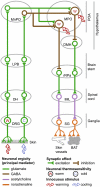
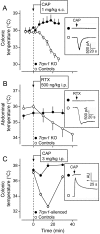
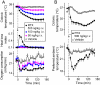
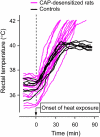
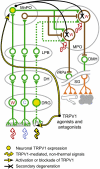
The development of antagonists of the transient receptor potential vanilloid-1 (TRPV1) channel as pain therapeutics has revealed that these compounds cause hyperthermia in humans. This undesirable on-target side effect has triggered a surge of interest in the role of TRPV1 in thermoregulation and revived the hypothesis that TRPV1 channels serve as thermosensors. We review literature data on the distribution of TRPV1 channels in the body and on thermoregulatory responses to TRPV1 agonists and antagonists. We propose that two principal populations of TRPV1-expressing cells have connections with efferent thermoeffector pathways: 1) first-order sensory (polymodal), glutamatergic dorsal-root (and possibly nodose) ganglia neurons that innervate the abdominal viscera and 2) higher-order sensory, glutamatergic neurons presumably located in the median preoptic hypothalamic nucleus. We further hypothesize that all thermoregulatory responses to TRPV1 agonists and antagonists and thermoregulatory manifestations of TRPV1 desensitization stem from primary actions on these two neuronal populations. Agonists act primarily centrally on population 2; antagonists act primarily peripherally on population 1. We analyze what roles TRPV1 might play in thermoregulation and conclude that this channel does not serve as a thermosensor, at least not under physiological conditions. In the hypothalamus, TRPV1 channels are inactive at common brain temperatures. In the abdomen, TRPV1 channels are tonically activated, but not by temperature. However, tonic activation of visceral TRPV1 by nonthermal factors suppresses autonomic cold-defense effectors and, consequently, body temperature. Blockade of this activation by TRPV1 antagonists disinhibits thermoeffectors and causes hyperthermia. Strategies for creating hyperthermia-free TRPV1 antagonists are outlined. The potential physiological and pathological significance of TRPV1-mediated thermoregulatory effects is discussed.
Figures


Comment in
-
Answering the burning question of how transient receptor potential vanilloid-1 channel antagonists cause unwanted hyperthermia.Pharmacol Rev. 2009 Sep;61(3):225-7. doi: 10.1124/pr.109.001875. Pharmacol Rev. 2009. PMID: 19805475
Similar articles
-
Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors.J Neurosci. 2007 Jul 11;27(28):7459-68. doi: 10.1523/JNEUROSCI.1483-07.2007. J Neurosci. 2007. PMID: 17626206 Free PMC article.
-
Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis.Pharmacol Ther. 2020 Apr;208:107474. doi: 10.1016/j.pharmthera.2020.107474. Epub 2020 Jan 9. Pharmacol Ther. 2020. PMID: 31926897 Review.
-
The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation.J Neurosci. 2007 Mar 28;27(13):3366-74. doi: 10.1523/JNEUROSCI.4833-06.2007. J Neurosci. 2007. PMID: 17392452 Free PMC article.
-
Answering the burning question of how transient receptor potential vanilloid-1 channel antagonists cause unwanted hyperthermia.Pharmacol Rev. 2009 Sep;61(3):225-7. doi: 10.1124/pr.109.001875. Pharmacol Rev. 2009. PMID: 19805475
-
The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor.Br J Pharmacol. 2008 Dec;155(8):1145-62. doi: 10.1038/bjp.2008.351. Epub 2008 Sep 22. Br J Pharmacol. 2008. PMID: 18806809 Free PMC article. Review.
Cited by
-
Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold.Neuron. 2013 Apr 10;78(1):138-51. doi: 10.1016/j.neuron.2013.01.030. Epub 2013 Mar 21. Neuron. 2013. PMID: 23523592 Free PMC article.
-
Nociception and pain in humans lacking a functional TRPV1 channel.J Clin Invest. 2023 Feb 1;133(3):e153558. doi: 10.1172/JCI153558. J Clin Invest. 2023. PMID: 36454632 Free PMC article.
-
Integration of thermal and osmotic regulation of water homeostasis: the role of TRPV channels.Am J Physiol Regul Integr Comp Physiol. 2013 Oct 1;305(7):R669-78. doi: 10.1152/ajpregu.00270.2013. Epub 2013 Jul 24. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23883678 Free PMC article. Review.
-
Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance.FASEB J. 2015 Aug;29(8):3182-92. doi: 10.1096/fj.14-268300. Epub 2015 Apr 17. FASEB J. 2015. PMID: 25888600 Free PMC article.
-
Thermoregulatory and cardiovasculareffects of capsaicin application on human skin during dynamic exercise to temperate and warm conditions.Physiol Rep. 2019 Dec;7(24):e14325. doi: 10.14814/phy2.14325. Physiol Rep. 2019. PMID: 31883232 Free PMC article.
References
-
- Acs G, Palkovits M, Blumberg PM. (1995) Trifluoperazine modulates [3H]resiniferatoxin binding by human and rat vanilloid (capsaicin) receptors and affects 45Ca uptake by adult rat dorsal root ganglion neurones. J Pharmacol Exp Ther .274: 1090–1098 - PubMed
-
- Acs G, Palkovits M, Blumberg PM. (1996) Specific binding of [3H]resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sci .59: 1899–1908 - PubMed
-
- Adams IB, Compton DR, Martin BR. (1998) Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther .284: 1209–1217 - PubMed
-
- Adelson DW, Wei JY, Kruger L. (1997) Warm-sensitive afferent splanchnic C-fiber units in vitro. J Neurophysiol .77: 2989–3002 - PubMed
-
- Ahern GP. (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem .278: 30429–30434 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
