Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens
- PMID: 19748484
- PMCID: PMC2787893
- DOI: 10.1016/j.bbrc.2009.09.029
Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens
Abstract
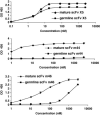
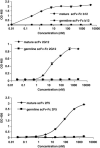
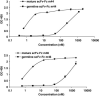
Several human monoclonal antibodies (hmAbs) including b12, 2G12, and 2F5 exhibit relatively potent and broad HIV-1-neutralizing activity. However, their elicitation in vivo by vaccine immunogens based on the HIV-1 envelope glycoprotein (Env) has not been successful. We have hypothesized that HIV-1 has evolved a strategy to reduce or eliminate the immunogenicity of the highly conserved epitopes of such antibodies by using "holes" (absence or very weak binding to these epitopes of germline antibodies that is not sufficient to initiate and/or maintain an efficient immune response) in the human germline B cell receptor (BCR) repertoire. To begin to test this hypothesis we have designed germline-like antibodies corresponding most closely to b12, 2G12, and 2F5 as well as to X5, m44, and m46 which are cross-reactive but with relatively modest neutralizing activity as natively occurring antibodies due to size and/or other effects. The germline-like X5, m44, and m46 bound with relatively high affinity to all tested Envs. In contrast, germline-like b12, 2G12, and 2F5 lacked measurable binding to Envs in an ELISA assay although the corresponding mature antibodies did. These results provide initial evidence that Env structures containing conserved vulnerable epitopes may not initiate humoral responses by binding to germline antibodies. Even if such responses are initiated by very weak binding undetectable in our assay it is likely that they will be outcompeted by responses to structures containing the epitopes of X5, m44, m46, and other antibodies that bind germline BCRs with much higher affinity/avidity. This hypothesis, if further supported by data, could contribute to our understanding of how HIV-1 evades immune responses and offer new concepts for design of effective vaccine immunogens.
Figures

Similar articles
-
Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D.J Virol. 2014 Mar;88(5):2645-57. doi: 10.1128/JVI.03228-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352455 Free PMC article.
-
A single mutation turns a non-binding germline-like predecessor of broadly neutralizing antibody into a binding antibody to HIV-1 envelope glycoproteins.MAbs. 2011 Jul-Aug;3(4):402-7. doi: 10.4161/mabs.3.4.15740. Epub 2011 Jul 1. MAbs. 2011. PMID: 21540646 Free PMC article.
-
Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs.PLoS Pathog. 2013 Jan;9(1):e1003106. doi: 10.1371/journal.ppat.1003106. Epub 2013 Jan 3. PLoS Pathog. 2013. PMID: 23300456 Free PMC article.
-
Neutralizing antibodies and control of HIV: moves and countermoves.Curr HIV/AIDS Rep. 2012 Mar;9(1):64-72. doi: 10.1007/s11904-011-0105-5. Curr HIV/AIDS Rep. 2012. PMID: 22203469 Review.
-
HIV-1 envelope glycoprotein immunogens to induce broadly neutralizing antibodies.Expert Rev Vaccines. 2016;15(3):349-65. doi: 10.1586/14760584.2016.1129905. Epub 2016 Jan 8. Expert Rev Vaccines. 2016. PMID: 26654478 Review.
Cited by
-
Sequential and Simultaneous Immunization of Rabbits with HIV-1 Envelope Glycoprotein SOSIP.664 Trimers from Clades A, B and C.PLoS Pathog. 2016 Sep 14;12(9):e1005864. doi: 10.1371/journal.ppat.1005864. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27627672 Free PMC article.
-
Approaches to the induction of HIV broadly neutralizing antibodies.Curr Opin HIV AIDS. 2016 Nov;11(6):569-575. doi: 10.1097/COH.0000000000000317. Curr Opin HIV AIDS. 2016. PMID: 27559709 Free PMC article. Review.
-
Virological features associated with the development of broadly neutralizing antibodies to HIV-1.Trends Microbiol. 2015 Apr;23(4):204-11. doi: 10.1016/j.tim.2014.12.007. Epub 2015 Jan 5. Trends Microbiol. 2015. PMID: 25572881 Free PMC article. Review.
-
Conformational epitope consisting of the V3 and V4 loops as a target for potent and broad neutralization of simian immunodeficiency viruses.J Virol. 2013 May;87(10):5424-36. doi: 10.1128/JVI.00201-13. Epub 2013 Mar 6. J Virol. 2013. PMID: 23468483 Free PMC article.
-
Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques.J Exp Med. 2014 Sep 22;211(10):2061-74. doi: 10.1084/jem.20132494. Epub 2014 Aug 25. J Exp Med. 2014. PMID: 25155019 Free PMC article.
References
-
- Johnson W.E., Desrosiers R.C. Viral persistence: HIV’s strategies of immune system evasion. Annu. Rev. Med. 2002;53:499–518. - PubMed
-
- Wei X., Decker J.M., Wang S., Hui H., Kappes J.C., Wu X., Salazar-Gonzalez J.F., Salazar M.G., Kilby J.M., Saag M.S., Komarova N.L., Nowak M.A., Hahn B.H., Kwong P.D., Shaw G.M. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
-
- Burton D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2002;2:706–713. - PubMed
-
- Poignard P., Saphire E.O., Parren P.W., Burton D.R. Gp120: biologic aspects of structural features. Annu. Rev. Immunol. 2001;19:253–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

