Sumoylation of forkhead L2 by Ubc9 is required for its activity as a transcriptional repressor of the Steroidogenic Acute Regulatory gene
- PMID: 19744555
- PMCID: PMC2830813
- DOI: 10.1016/j.cellsig.2009.09.001
Sumoylation of forkhead L2 by Ubc9 is required for its activity as a transcriptional repressor of the Steroidogenic Acute Regulatory gene
Abstract
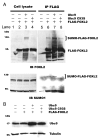
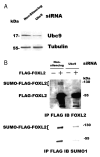
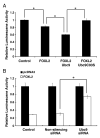
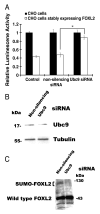
Forkhead L2 (FOXL2) is a member of the forkhead/hepatocyte nuclear factor 3 (FKH/HNF3) gene family of transcription factors and acts as a transcriptional repressor of the Steroidogenic Acute Regulatory (StAR) gene, a marker of granulosa cell differentiation. FOXL2 may play a role in ovarian follicle maturation and prevent premature follicle depletion leading to premature ovarian failure. In this study, we found that FOXL2 interacts with Ubc9, an E2-conjugating enzyme that mediates sumoylation, a key mechanism in transcriptional regulation. FOXL2 and Ubc9 are co-expressed in granulosa cells of small and medium ovarian follicles. FOXL2 is sumoylated by Ubc9, and this Ubc9-mediated sumoylation is essential to the transcriptional activity of FOXL2 on the StAR promoter. As FOXL2 is endogenous to granulosa cells, we generated a stable cell line expressing FOXL2 and found that activity of the StAR promoter in this cell line is greatly decreased in the presence of Ubc9. The sumoylation site was identified at lysine 25 of FOXL2. Mutation of lysine 25 to arginine leads to loss of transcriptional repressor activity of FOXL2. Taken together, we propose that Ubc9-mediated sumoylation at lysine 25 of FOXL2 is required for transcriptional repression of the StAR gene and may be responsible for controlling the development of ovarian follicles.
Conflict of interest statement
Figures




Similar articles
-
LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity.Am J Physiol Endocrinol Metab. 2010 Jul;299(1):E101-9. doi: 10.1152/ajpendo.00534.2009. Epub 2010 Apr 20. Am J Physiol Endocrinol Metab. 2010. PMID: 20407010 Free PMC article.
-
Mouse forkhead L2 maintains repression of FSH-dependent genes in the granulosa cell.Reproduction. 2012 Oct;144(4):485-94. doi: 10.1530/REP-11-0259. Epub 2012 Jul 30. Reproduction. 2012. PMID: 22847492
-
Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene.Endocrinology. 2004 Jul;145(7):3424-33. doi: 10.1210/en.2003-1141. Epub 2004 Apr 1. Endocrinology. 2004. PMID: 15059956
-
Minireview: roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology.Endocrinology. 2011 Apr;152(4):1199-208. doi: 10.1210/en.2010-1041. Epub 2011 Jan 19. Endocrinology. 2011. PMID: 21248146 Free PMC article. Review.
-
FOXL2: a central transcription factor of the ovary.J Mol Endocrinol. 2013 Dec 19;52(1):R17-33. doi: 10.1530/JME-13-0159. Print 2014 Feb. J Mol Endocrinol. 2013. PMID: 24049064 Review.
Cited by
-
Synergistic activation of the Mc2r promoter by FOXL2 and NR5A1 in mice.Biol Reprod. 2010 Nov;83(5):842-51. doi: 10.1095/biolreprod.110.085621. Epub 2010 Jul 21. Biol Reprod. 2010. PMID: 20650879 Free PMC article.
-
The Hippo/MST Pathway Member SAV1 Plays a Suppressive Role in Development of the Prehierarchical Follicles in Hen Ovary.PLoS One. 2016 Aug 9;11(8):e0160896. doi: 10.1371/journal.pone.0160896. eCollection 2016. PLoS One. 2016. PMID: 27505353 Free PMC article.
-
Mutant Forkhead L2 (FOXL2) proteins associated with premature ovarian failure (POF) dimerize with wild-type FOXL2, leading to altered regulation of genes associated with granulosa cell differentiation.Endocrinology. 2011 Oct;152(10):3917-29. doi: 10.1210/en.2010-0989. Epub 2011 Aug 23. Endocrinology. 2011. PMID: 21862621 Free PMC article.
-
LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity.Am J Physiol Endocrinol Metab. 2010 Jul;299(1):E101-9. doi: 10.1152/ajpendo.00534.2009. Epub 2010 Apr 20. Am J Physiol Endocrinol Metab. 2010. PMID: 20407010 Free PMC article.
-
The forkhead transcription factor Foxl2 is sumoylated in both human and mouse: sumoylation affects its stability, localization, and activity.PLoS One. 2010 Mar 2;5(3):e9477. doi: 10.1371/journal.pone.0009477. PLoS One. 2010. PMID: 20209145 Free PMC article.
References
-
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. Nat Genet. 2001 Feb;27(2):159–166. - PubMed
-
- Weigel D, Jurgens G, Kuttner F, Seifert E. Jackle H Cell. 1989;57(4):645–658. - PubMed
-
- Matsuzaki K, Minami T, Tojo M, Honda Y, Uchimura Y, Saitoh H, Yasuda H, Nagahiro S, Saya H, Nakao M. Biochem Biophys Res Commun. 2003 Jun 20;306(1):32–38. - PubMed
-
- Parry P, Wei Y, Evans G. Genes Chromosomes Cancer. 1994;11(2):79–84. - PubMed
-
- Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. Cancer Res. 2002;62(16):4773–4780. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

