Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance
- PMID: 19741708
- PMCID: PMC2851546
- DOI: 10.1038/nature08296
Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance
Abstract
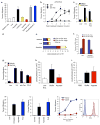
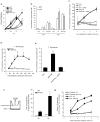
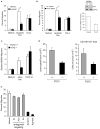
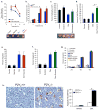
Phagocytic removal of apoptotic cells occurs efficiently in vivo such that even in tissues with significant apoptosis, very few apoptotic cells are detectable. This is thought to be due to the release of 'find-me' signals by apoptotic cells that recruit motile phagocytes such as monocytes, macrophages and dendritic cells, leading to the prompt clearance of the dying cells. However, the identity and in vivo relevance of such find-me signals are not well understood. Here, through several lines of evidence, we identify extracellular nucleotides as a critical apoptotic cell find-me signal. We demonstrate the caspase-dependent release of ATP and UTP (in equimolar quantities) during the early stages of apoptosis by primary thymocytes and cell lines. Purified nucleotides at these concentrations were sufficient to induce monocyte recruitment comparable to that of apoptotic cell supernatants. Enzymatic removal of ATP and UTP (by apyrase or the expression of ectopic CD39) abrogated the ability of apoptotic cell supernatants to recruit monocytes in vitro and in vivo. We then identified the ATP/UTP receptor P2Y(2) as a critical sensor of nucleotides released by apoptotic cells using RNA interference-mediated depletion studies in monocytes, and macrophages from P2Y(2)-null mice. The relevance of nucleotides in apoptotic cell clearance in vivo was revealed by two approaches. First, in a murine air-pouch model, apoptotic cell supernatants induced a threefold greater recruitment of monocytes and macrophages than supernatants from healthy cells did; this recruitment was abolished by depletion of nucleotides and was significantly decreased in P2Y(2)(-/-) (also known as P2ry2(-/-)) mice. Second, clearance of apoptotic thymocytes was significantly impaired by either depletion of nucleotides or interference with P2Y receptor function (by pharmacological inhibition or in P2Y(2)(-/-) mice). These results identify nucleotides as a critical find-me cue released by apoptotic cells to promote P2Y(2)-dependent recruitment of phagocytes, and provide evidence for a clear relationship between a find-me signal and efficient corpse clearance in vivo.
Figures
Comment in
-
Cell biology: Sent by the scent of death.Nature. 2009 Sep 10;461(7261):181-2. doi: 10.1038/461181a. Nature. 2009. PMID: 19741694 No abstract available.
Similar articles
-
Cell biology: Sent by the scent of death.Nature. 2009 Sep 10;461(7261):181-2. doi: 10.1038/461181a. Nature. 2009. PMID: 19741694 No abstract available.
-
Autocrine purinergic receptor signaling is essential for macrophage chemotaxis.Sci Signal. 2010 Jul 27;3(132):ra55. doi: 10.1126/scisignal.2000588. Sci Signal. 2010. PMID: 20664064
-
Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis.Nature. 2010 Oct 14;467(7317):863-7. doi: 10.1038/nature09413. Nature. 2010. PMID: 20944749 Free PMC article.
-
Do not let death do us part: 'find-me' signals in communication between dying cells and the phagocytes.Cell Death Differ. 2016 Jun;23(6):979-89. doi: 10.1038/cdd.2016.13. Epub 2016 Feb 19. Cell Death Differ. 2016. PMID: 26891690 Free PMC article. Review.
-
Surface code--biophysical signals for apoptotic cell clearance.Phys Biol. 2013 Dec;10(6):065007. doi: 10.1088/1478-3975/10/6/065007. Epub 2013 Dec 4. Phys Biol. 2013. PMID: 24305041 Review.
Cited by
-
How neutrophil extracellular traps orchestrate the local immune response in gout.J Mol Med (Berl). 2015 Jul;93(7):727-34. doi: 10.1007/s00109-015-1295-x. Epub 2015 May 24. J Mol Med (Berl). 2015. PMID: 26002146 Review.
-
P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo.Purinergic Signal. 2013 Dec;9(4):633-42. doi: 10.1007/s11302-013-9374-3. Epub 2013 Jul 5. Purinergic Signal. 2013. PMID: 23828651 Free PMC article.
-
Piezo1 expression in neutrophils regulates shear-induced NETosis.Nat Commun. 2024 Aug 22;15(1):7023. doi: 10.1038/s41467-024-51211-1. Nat Commun. 2024. PMID: 39174529 Free PMC article.
-
Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection.Diabetes. 2013 May;62(5):1665-75. doi: 10.2337/db12-0242. Epub 2013 Jan 11. Diabetes. 2013. PMID: 23315496 Free PMC article.
-
Photoacoustic mediated multifunctional tumor antigen trapping nanoparticles inhibit the recurrence and metastasis of ovarian cancer by enhancing tumor immunogenicity.J Nanobiotechnology. 2022 Nov 3;20(1):468. doi: 10.1186/s12951-022-01682-5. J Nanobiotechnology. 2022. PMID: 36329515 Free PMC article.
References
-
- Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. - PubMed
-
- Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. - PubMed
-
- Homolya L, Watt WC, Lazarowski ER, Koller BH, Boucher RC. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. J Biol Chem. 1999;274:26454–26460. - PubMed
-
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. - PubMed
-
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

