Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins
- PMID: 19737820
- PMCID: PMC2746133
- DOI: 10.1242/jcs.034298
Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins
Abstract
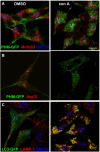
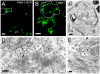
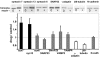
The vacuolar H(+)-ATPase (V-ATPase) establishes pH gradients along secretory and endocytic pathways. Progressive acidification is essential for proteolytic processing of prohormones and aggregation of soluble content proteins. The V-ATPase V(0) subunit is thought to have a separate role in budding and fusion events. Prolonged treatment of professional secretory cells with selective V-ATPase inhibitors (bafilomycin A1, concanamycin A) was used to investigate its role in secretory-granule biogenesis. As expected, these inhibitors eliminated regulated secretion and blocked prohormone processing. Drug treatment caused the formation of large, mixed organelles, with components of immature granules and lysosomes and some markers of autophagy. Markers of the trans-Golgi network and earlier secretory pathway were unaffected. Ammonium chloride and methylamine treatment blocked acidification to a similar extent as the V-ATPase inhibitors without producing mixed organelles. Newly synthesized granule content proteins appeared in mixed organelles, whereas mature secretory granules were spared. Following concanamycin treatment, selected membrane proteins enter tubulovesicular structures budding into the interior of mixed organelles. shRNA-mediated knockdown of the proteolipid subunit of V(0) also caused vesiculation of immature granules. Thus, V-ATPase has a role in protein sorting in immature granules that is distinct from its role in acidification.
Figures
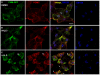
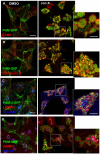
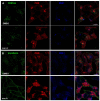
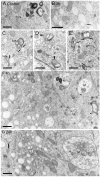



Similar articles
-
The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases.Transl Neurodegener. 2020 May 11;9(1):17. doi: 10.1186/s40035-020-00196-0. Transl Neurodegener. 2020. PMID: 32393395 Free PMC article. Review.
-
Proton pumping V-ATPase inhibitor bafilomycin A1 affects Rab7 lysosomal localization and abolishes anterograde trafficking of osteoclast secretory lysosomes.Biochem Biophys Res Commun. 2019 Mar 12;510(3):421-426. doi: 10.1016/j.bbrc.2019.01.118. Epub 2019 Feb 1. Biochem Biophys Res Commun. 2019. PMID: 30717974
-
Role of H+-ATPase-mediated acidification in sorting and release of the regulated secretory protein chromogranin A: evidence for a vesiculogenic function.J Biol Chem. 2005 Feb 4;280(5):3885-97. doi: 10.1074/jbc.M408197200. Epub 2004 Nov 12. J Biol Chem. 2005. PMID: 15542860
-
Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate-specific antigen expression and secretion.Int J Cancer. 2013 Jan 15;132(2):E1-10. doi: 10.1002/ijc.27811. Epub 2012 Sep 21. Int J Cancer. 2013. PMID: 22945374 Free PMC article.
-
Vacuolar-type ATPase: A proton pump to lysosomal trafficking.Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(6):261-277. doi: 10.2183/pjab.95.018. Proc Jpn Acad Ser B Phys Biol Sci. 2019. PMID: 31189779 Free PMC article. Review.
Cited by
-
58-F, a flavanone from Ophiopogon japonicus, prevents hepatocyte death by decreasing lysosomal membrane permeability.Sci Rep. 2016 Jun 16;6:27875. doi: 10.1038/srep27875. Sci Rep. 2016. PMID: 27306715 Free PMC article.
-
A pH-sensitive luminal His-cluster promotes interaction of PAM with V-ATPase along the secretory and endocytic pathways of peptidergic cells.J Cell Physiol. 2019 Jun;234(6):8683-8697. doi: 10.1002/jcp.27528. Epub 2018 Oct 14. J Cell Physiol. 2019. PMID: 30317586 Free PMC article.
-
The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases.Transl Neurodegener. 2020 May 11;9(1):17. doi: 10.1186/s40035-020-00196-0. Transl Neurodegener. 2020. PMID: 32393395 Free PMC article. Review.
-
Both Matricaria chamomilla and Metformin Extract Improved the Function and Histological Structure of Thyroid Gland in Polycystic Ovary Syndrome Rats through Antioxidant Mechanism.Biomolecules. 2020 Jan 5;10(1):88. doi: 10.3390/biom10010088. Biomolecules. 2020. PMID: 31948119 Free PMC article.
-
Functional vacuolar ATPase (V-ATPase) proton pumps traffic to the enterocyte brush border membrane and require CFTR.Am J Physiol Cell Physiol. 2013 Nov 1;305(9):C981-96. doi: 10.1152/ajpcell.00067.2013. Epub 2013 Aug 28. Am J Physiol Cell Physiol. 2013. PMID: 23986201 Free PMC article.
References
-
- Back, N. and Soinila, S. (1996). Dose-dependent effects of chloroquine on secretory granule formation in the melanotroph. Acta Anat. 156, 307-314. - PubMed
-
- Beyenbach, K. W. and Wieczorek, H. (2006). The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577-589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

