Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1
- PMID: 19736547
- PMCID: PMC2783367
- DOI: 10.1038/labinvest.2009.93
Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1
Abstract
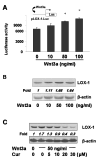
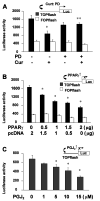
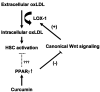
Type II diabetes mellitus (T2DM) is often accompanied by non-alcoholic steatohepatitis (NASH) and associated with hypercholesterolemia, that is, increased levels of plasma low-density lipoprotein (LDL) and oxidized LDL (ox-LDL). Approximately one-third of NASH develops hepatic fibrosis. The role of hypercholesterolemia in T2DM and NASH-associated hepatic fibrogenesis remains obscure. We previously reported that the phytochemical curcumin inhibited the activation of hepatic stellate cells (HSCs), the major effector cells during hepatic fibrogenesis, and protected the liver from fibrogenesis in vitro and in vivo. The aims of this study are to evaluate the role of ox-LDL in activation of HSCs, to assess curcumin effects on eliminating the role of ox-LDL, and to further explore the underlying mechanisms. In this report, we observe that ox-LDL alters the expression of genes closely relevant to HSC activation, which is eliminated by curcumin. Curcumin suppresses gene expression of lectin-like oxidized LDL receptor-1 (LOX-1), leading to the blockade of the transport of extracellular ox-LDL into cells. This suppressive effect of curcumin results from the interruption of Wnt signaling and the activation of peroxisome proliferator-activated receptor-gamma (PPARgamma). In conclusion, these results support our initial hypothesis and demonstrate that ox-LDL stimulates HSC activation, which is eliminated by curcumin by suppressing lox-1 expression by interrupting Wnt signaling and stimulating PPARgamma activity. These results provide novel insights into the role of ox-LDL in T2DM and NASH-associated hepatic fibrogenesis and mechanisms by which curcumin suppresses ox-LDL-induced HSC activation, as well as the implication of curcumin in the treatment of T2DM and NASH-associated hepatic fibrosis.
Conflict of interest statement
Figures
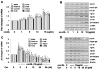
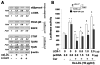
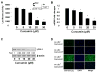
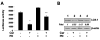



Comment in
-
Inside lab invest.Lab Invest. 2009 Nov;89(11):1190-1. doi: 10.1038/labinvest.2009.114. Lab Invest. 2009. PMID: 19861966 No abstract available.
Similar articles
-
Curcumin suppresses expression of low-density lipoprotein (LDL) receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells.Br J Pharmacol. 2009 Aug;157(8):1354-67. doi: 10.1111/j.1476-5381.2009.00261.x. Epub 2009 Jul 7. Br J Pharmacol. 2009. PMID: 19594758 Free PMC article.
-
Curcumin eliminates leptin's effects on hepatic stellate cell activation via interrupting leptin signaling.Endocrinology. 2009 Jul;150(7):3011-20. doi: 10.1210/en.2008-1601. Epub 2009 Mar 19. Endocrinology. 2009. PMID: 19299451 Free PMC article.
-
Curcumin diminishes the impacts of hyperglycemia on the activation of hepatic stellate cells by suppressing membrane translocation and gene expression of glucose transporter-2.Mol Cell Endocrinol. 2011 Feb 20;333(2):160-71. doi: 10.1016/j.mce.2010.12.028. Epub 2010 Dec 30. Mol Cell Endocrinol. 2011. PMID: 21195127 Free PMC article.
-
Wnt signaling in liver fibrosis: progress, challenges and potential directions.Biochimie. 2013 Dec;95(12):2326-35. doi: 10.1016/j.biochi.2013.09.003. Epub 2013 Sep 13. Biochimie. 2013. PMID: 24036368 Review.
-
Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo.Dig Dis Sci. 2015 Jun;60(6):1554-64. doi: 10.1007/s10620-014-3487-6. Epub 2014 Dec 23. Dig Dis Sci. 2015. PMID: 25532502 Review.
Cited by
-
Perilipin 5 and liver fatty acid binding protein function to restore quiescence in mouse hepatic stellate cells.J Lipid Res. 2018 Mar;59(3):416-428. doi: 10.1194/jlr.M077487. Epub 2018 Jan 9. J Lipid Res. 2018. PMID: 29317465 Free PMC article.
-
Curcumin Alleviates Palmitic Acid-Induced LOX-1 Upregulation by Suppressing Endoplasmic Reticulum Stress in HUVECs.Biomed Res Int. 2021 Aug 22;2021:9983725. doi: 10.1155/2021/9983725. eCollection 2021. Biomed Res Int. 2021. PMID: 34471643 Free PMC article.
-
Therapeutic potential of curcumin in gastrointestinal diseases.World J Gastrointest Pathophysiol. 2011 Feb 15;2(1):1-14. doi: 10.4291/wjgp.v2.i1.1. World J Gastrointest Pathophysiol. 2011. PMID: 21607160 Free PMC article.
-
Biology of pancreatic stellate cells-more than just pancreatic cancer.Pflugers Arch. 2017 Sep;469(9):1039-1050. doi: 10.1007/s00424-017-1968-0. Epub 2017 Apr 5. Pflugers Arch. 2017. PMID: 28382480 Free PMC article. Review.
-
Curcumin and diabetes: a systematic review.Evid Based Complement Alternat Med. 2013;2013:636053. doi: 10.1155/2013/636053. Epub 2013 Nov 24. Evid Based Complement Alternat Med. 2013. PMID: 24348712 Free PMC article. Review.
References
-
- Tsochatzis E, Papatheodoridis GV, Manesis EK, Kafiri G, Tiniakos DG, Archimandritis AJ. Metabolic syndrome is associated with severe fibrosis in chronic viral hepatitis and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;27:80–89. - PubMed
-
- Jacqueminet S, Lebray P, Morra R, Munteanu M, Devers L, Messous D, Bernard M, Hartemann-Heurtier A, Imbert-Bismut F, Ratziu V, Grimaldi A, Poynard T. Screening for liver fibrosis by using a noninvasive biomarker in patients with diabetes. Clin Gastroenterol Hepatol. 2008;6:828–831. - PubMed
-
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(1):S5–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

