Helicase-appended topoisomerases: new insight into the mechanism of directional strand transfer
- PMID: 19726668
- PMCID: PMC2781472
- DOI: 10.1074/jbc.R109.051268
Helicase-appended topoisomerases: new insight into the mechanism of directional strand transfer
Abstract
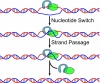
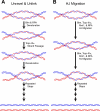
DNA strand passage through an enzyme-mediated gate is a key step in the catalytic cycle of topoisomerases to produce topological transformations in DNA. In most of the reactions catalyzed by topoisomerases, strand passage is not directional; thus, the enzyme simply provides a transient DNA gate through which DNA transport is allowed and thereby resolves the topological entanglement. When studied in isolation, the type IA topoisomerase family appears to conform to this rule. Interestingly, type IA enzymes can carry out directional strand transport as well. We examined here the biochemical mechanism for directional strand passage of two type IA topoisomerases: reverse gyrase and a protein complex of topoisomerase III alpha and Bloom helicase. These enzymes are able to generate vectorial strand transport independent of the supercoiling energy stored in the DNA molecule. Reverse gyrase is able to anneal single strands, thereby increasing linkage number of a DNA molecule. However, topoisomerase III alpha and Bloom helicase can dissolve DNA conjoined with a double Holliday junction, thus reducing DNA linkage. We propose here that the helicase or helicase-like component plays a determinant role in the directionality of strand transport. There is thus a common biochemical ground for the directional strand passage for the type IA topoisomerases.
Figures
Similar articles
-
Direct observation of helicase-topoisomerase coupling within reverse gyrase.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10856-10864. doi: 10.1073/pnas.1921848117. Epub 2020 May 5. Proc Natl Acad Sci U S A. 2020. PMID: 32371489 Free PMC article.
-
What's on the Other Side of the Gate: A Structural Perspective on DNA Gate Opening of Type IA and IIA DNA Topoisomerases.Int J Mol Sci. 2023 Feb 16;24(4):3986. doi: 10.3390/ijms24043986. Int J Mol Sci. 2023. PMID: 36835394 Free PMC article. Review.
-
The mechanism of negative DNA supercoiling: a cascade of DNA-induced conformational changes prepares gyrase for strand passage.DNA Repair (Amst). 2014 Apr;16:23-34. doi: 10.1016/j.dnarep.2014.01.011. Epub 2014 Feb 22. DNA Repair (Amst). 2014. PMID: 24674625 Review.
-
Reprint of "The mechanism of negative DNA supercoiling: a cascade of DNA-induced conformational changes prepares gyrase for strand passage".DNA Repair (Amst). 2014 Aug;20:130-141. doi: 10.1016/j.dnarep.2014.06.006. Epub 2014 Jun 25. DNA Repair (Amst). 2014. PMID: 24974097
-
Synthesis and dissolution of hemicatenanes by type IA DNA topoisomerases.Proc Natl Acad Sci U S A. 2013 Sep 17;110(38):E3587-94. doi: 10.1073/pnas.1304103110. Epub 2013 Sep 3. Proc Natl Acad Sci U S A. 2013. PMID: 24003117 Free PMC article.
Cited by
-
Direct observation of DNA overwinding by reverse gyrase.Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7495-500. doi: 10.1073/pnas.1422203112. Epub 2015 May 28. Proc Natl Acad Sci U S A. 2015. PMID: 26023188 Free PMC article.
-
The many lives of type IA topoisomerases.J Biol Chem. 2020 May 15;295(20):7138-7153. doi: 10.1074/jbc.REV120.008286. Epub 2020 Apr 10. J Biol Chem. 2020. PMID: 32277049 Free PMC article. Review.
-
Genome stability: recent insights in the topoisomerase reverse gyrase and thermophilic DNA alkyltransferase.Extremophiles. 2014 Sep;18(5):895-904. doi: 10.1007/s00792-014-0662-9. Epub 2014 Aug 8. Extremophiles. 2014. PMID: 25102812 Review.
-
Synergic and opposing activities of thermophilic RecQ-like helicase and topoisomerase 3 proteins in Holliday junction processing and replication fork stabilization.J Biol Chem. 2012 Aug 31;287(36):30282-95. doi: 10.1074/jbc.M112.366377. Epub 2012 Jun 21. J Biol Chem. 2012. PMID: 22722926 Free PMC article.
-
Top3α is required during the convergent migration step of double Holliday junction dissolution.PLoS One. 2014 Jan 2;9(1):e83582. doi: 10.1371/journal.pone.0083582. eCollection 2014. PLoS One. 2014. PMID: 24392087 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

