Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2
- PMID: 19726544
- PMCID: PMC2781345
- DOI: 10.1152/ajprenal.00412.2009
Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2
Abstract
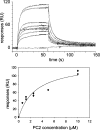
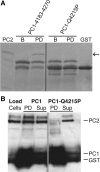
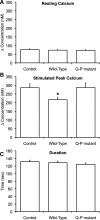
Autosomal dominant polycystic kidney disease (ADPKD) arises following mutations of either Pkd1 or Pkd2. The proteins these genes encode, polycystin-1 (PC1) and polycystin-2 (PC2), form a signaling complex using direct intermolecular interactions. Two distinct domains in the C-terminal tail of PC2 have recently been identified, an EF-hand and a coiled-coil domain. Here, we show that the PC2 coiled-coil domain interacts with the C-terminal tail of PC1, but that the PC2 EF-hand domain does not. We measured the K0.5 of the interaction between the C-terminal tails of PC1 and PC2 and showed that the direct interaction of these proteins is abrogated by a PC1 point mutation that was identified in ADPKD patients. Finally, we showed that overexpression of the PC1 C-terminal tail in MDCK cells alters the Ca2+ response, but that overexpression of the PC1 C-terminal tail containing the disease mutation does not. These results allow a more detailed understanding of the mechanism of pathogenic mutations in the cytoplasmic regions of PC1 and PC2.
Figures

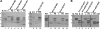
Similar articles
-
Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling.Am J Physiol Renal Physiol. 2007 Mar;292(3):F930-45. doi: 10.1152/ajprenal.00285.2006. Epub 2006 Nov 7. Am J Physiol Renal Physiol. 2007. PMID: 17090781 Free PMC article.
-
Polycystin-1 negatively regulates Polycystin-2 expression via the aggresome/autophagosome pathway.J Biol Chem. 2014 Mar 7;289(10):6404-6414. doi: 10.1074/jbc.M113.501205. Epub 2014 Jan 23. J Biol Chem. 2014. PMID: 24459142 Free PMC article.
-
Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner.J Clin Invest. 2015 Feb;125(2):607-20. doi: 10.1172/JCI76972. Epub 2015 Jan 9. J Clin Invest. 2015. PMID: 25574838 Free PMC article.
-
Genetic Mechanisms of ADPKD.Adv Exp Med Biol. 2016;933:13-22. doi: 10.1007/978-981-10-2041-4_2. Adv Exp Med Biol. 2016. PMID: 27730431 Review.
-
Polycystin 2: A calcium channel, channel partner, and regulator of calcium homeostasis in ADPKD.Cell Signal. 2020 Feb;66:109490. doi: 10.1016/j.cellsig.2019.109490. Epub 2019 Dec 2. Cell Signal. 2020. PMID: 31805375 Free PMC article. Review.
Cited by
-
Polycystin-1 surface localization is stimulated by polycystin-2 and cleavage at the G protein-coupled receptor proteolytic site.Mol Biol Cell. 2010 Dec;21(24):4338-48. doi: 10.1091/mbc.E10-05-0407. Epub 2010 Oct 27. Mol Biol Cell. 2010. PMID: 20980620 Free PMC article.
-
Polycystin-1 Assembles With Kv Channels to Govern Cardiomyocyte Repolarization and Contractility.Circulation. 2019 Sep 10;140(11):921-936. doi: 10.1161/CIRCULATIONAHA.118.034731. Epub 2019 Jun 21. Circulation. 2019. PMID: 31220931 Free PMC article.
-
Novel roles of Pkd2 in male reproductive system development.Differentiation. 2014 Mar-Apr;87(3-4):161-71. doi: 10.1016/j.diff.2014.04.001. Epub 2014 Jun 18. Differentiation. 2014. PMID: 24951251 Free PMC article.
-
Leucine-Rich Repeat in Polycystin-1 Suppresses Cystogenesis in a Zebrafish (Danio rerio) Model of Autosomal-Dominant Polycystic Kidney Disease.Int J Mol Sci. 2024 Mar 1;25(5):2886. doi: 10.3390/ijms25052886. Int J Mol Sci. 2024. PMID: 38474131 Free PMC article.
-
Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy.J Mol Cell Cardiol. 2013 May;58:199-208. doi: 10.1016/j.yjmcc.2013.01.015. Epub 2013 Jan 30. J Mol Cell Cardiol. 2013. PMID: 23376035 Free PMC article.
References
-
- Anyatonwu GI, Ehrlich BE. Organic cation permeation through the channel formed by polycystin-2. J Biol Chem 280: 29488–29493, 2005 - PubMed
-
- Badenas C, Torra R, San Millan JL, Lucero L, Mila M, Estivill X, Darnell A. Mutational analysis within the 3′ region of the PKD1 gene. Kidney Int 55: 1225–1233, 1999 - PubMed
-
- Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279: 19987–19995, 2004 - PubMed
-
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274: 28557–28565, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

