The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research
- PMID: 19723653
- PMCID: PMC5779623
- DOI: 10.1158/1078-0432.CCR-09-0737
The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research
Abstract
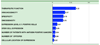
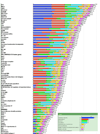
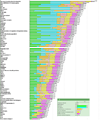
The purpose of the National Cancer Institute pilot project to prioritize cancer antigens was to develop a well-vetted, priority-ranked list of cancer vaccine target antigens based on predefined and preweighted objective criteria. An additional aim was for the National Cancer Institute to test a new approach for prioritizing translational research opportunities based on an analytic hierarchy process for dealing with complex decisions. Antigen prioritization involved developing a list of "ideal" cancer antigen criteria/characteristics, assigning relative weights to those criteria using pairwise comparisons, selecting 75 representative antigens for comparison and ranking, assembling information on the predefined criteria for the selected antigens, and ranking the antigens based on the predefined, preweighted criteria. Using the pairwise approach, the result of criteria weighting, in descending order, was as follows: (a) therapeutic function, (b) immunogenicity, (c) role of the antigen in oncogenicity, (d) specificity, (e) expression level and percent of antigen-positive cells, (f) stem cell expression, (g) number of patients with antigen-positive cancers, (h) number of antigenic epitopes, and (i) cellular location of antigen expression. None of the 75 antigens had all of the characteristics of the ideal cancer antigen. However, 46 were immunogenic in clinical trials and 20 of them had suggestive clinical efficacy in the "therapeutic function" category. These findings reflect the current status of the cancer vaccine field, highlight the possibility that additional organized efforts and funding would accelerate the development of therapeutically effective cancer vaccines, and accentuate the need for prioritization.
Figures
Comment in
-
Prioritization of cancer antigens: keeping the target in sight.Expert Rev Vaccines. 2009 Dec;8(12):1657-61. doi: 10.1586/erv.09.134. Expert Rev Vaccines. 2009. PMID: 19943761 No abstract available.
Similar articles
-
MAGE-A3: an immunogenic target used in clinical practice.Immunotherapy. 2015;7(6):683-704. doi: 10.2217/imt.15.29. Epub 2015 Jun 23. Immunotherapy. 2015. PMID: 26100270 Review.
-
Advances in the development of a therapeutic cancer vaccine.J Natl Compr Canc Netw. 2005 Nov;3 Suppl 1:S2-6. J Natl Compr Canc Netw. 2005. PMID: 16280106 No abstract available.
-
Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers.Immunol Invest. 2016 Oct;45(7):619-40. doi: 10.1080/08820139.2016.1197241. Epub 2016 Sep 7. Immunol Invest. 2016. PMID: 27603913 Review.
-
Cancer vaccines: an update with special focus on ganglioside antigens.Oncol Rep. 2002 Mar-Apr;9(2):267-76. Oncol Rep. 2002. PMID: 11836591 Review.
-
Antigen-specific vaccines for cancer treatment.Hum Vaccin Immunother. 2014;10(11):3332-46. doi: 10.4161/21645515.2014.973317. Hum Vaccin Immunother. 2014. PMID: 25483639 Free PMC article. Review.
Cited by
-
Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer.Prostate Cancer Prostatic Dis. 2013 Jun;16(2):123-31, S1. doi: 10.1038/pcan.2012.49. Epub 2013 Jan 8. Prostate Cancer Prostatic Dis. 2013. PMID: 23295316 Free PMC article.
-
New clinical advances in immunotherapy for the treatment of solid tumours.Immunology. 2015 Jun;145(2):182-201. doi: 10.1111/imm.12459. Epub 2015 Mar 30. Immunology. 2015. PMID: 25826229 Free PMC article. Review.
-
Analysis of CYP1B1 Polymorphisms in Lung Cancer Patients Using Novel, Quick and Easy Methods Based on CAPS and ACRS-PCR Techniques.Int J Mol Sci. 2024 Jun 18;25(12):6676. doi: 10.3390/ijms25126676. Int J Mol Sci. 2024. PMID: 38928381 Free PMC article.
-
Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy.Front Oncol. 2013 Mar 26;3:63. doi: 10.3389/fonc.2013.00063. eCollection 2013. Front Oncol. 2013. PMID: 23533029 Free PMC article.
-
The cancer glycocode as a family of diagnostic biomarkers, exemplified by tumor-associated gangliosides.Front Oncol. 2023 Oct 26;13:1261090. doi: 10.3389/fonc.2023.1261090. eCollection 2023. Front Oncol. 2023. PMID: 37954075 Free PMC article. Review.
References
-
- Oka Y, Tsuboi A, Oji Y, Kawase I, Sugiyama H. WT1 peptide vaccine for the treatment of cancer. Curr Opin Immunol. 2008;20:211–220. - PubMed
-
- Khanna R, Moss D, Gandhi M. Technology insight: Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat Clin Pract Oncol. 2005;2:138–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

