Protein kinase A and B-Raf mediate extracellular signal-regulated kinase activation by thyrotropin
- PMID: 19720729
- PMCID: PMC2774990
- DOI: 10.1124/mol.109.060129
Protein kinase A and B-Raf mediate extracellular signal-regulated kinase activation by thyrotropin
Abstract
Thyrotropin (TSH) regulates thyroid cell proliferation and function through cAMP-mediated signaling pathways that activate protein kinase A (PKA) and Epac/Rap1. The respective roles of PKA versus Epac/Rap1 in TSH signaling remain unclear. We set out to determine whether PKA and/or Rap1 mediate extracellular signal-regulated kinase (ERK) activation by TSH. Neither blocking Rap1 activity nor silencing the expression of Rap1 impaired TSH or forskolin-induced ERK activation in Wistar rat thyroid cells. Direct activation of Epac1 failed to stimulate ERK activity in starved cells, suggesting that Epac-induced Rap1 activity is not coupled to ERK activation in rat thyroid cells. By contrast, PKA activity was required for cAMP-stimulated ERK phosphorylation and was sufficient to increase ERK phosphorylation in starved cells. Expression of dominant-negative Ras inhibited ERK activation by TSH, forskolin, and N(6)-monobutyryl (6MB)-cAMP, a selective activator of PKA. Silencing the expression of B-Raf also inhibited ERK activation by TSH, forskolin, and 6MB-cAMP, but not that stimulated by insulin or serum. Depletion of B-Raf impaired TSH-induced DNA synthesis, indicating a functional role for B-Raf in TSH-regulated proliferation. Collectively, these results position PKA, Ras, and B-Raf as upstream regulators of ERK activation and identify B-Raf as a selective target of cAMP-elevating agents in thyroid cells. These data provide the first evidence for a functional role for B-Raf in TSH signaling.
Figures


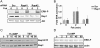

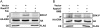

Similar articles
-
Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP.J Biol Chem. 2017 Jan 27;292(4):1449-1461. doi: 10.1074/jbc.M116.768986. Epub 2016 Dec 21. J Biol Chem. 2017. PMID: 28003362 Free PMC article.
-
Protein Kinase A-independent Ras Protein Activation Cooperates with Rap1 Protein to Mediate Activation of the Extracellular Signal-regulated Kinases (ERK) by cAMP.J Biol Chem. 2016 Oct 7;291(41):21584-21595. doi: 10.1074/jbc.M116.730978. Epub 2016 Aug 16. J Biol Chem. 2016. PMID: 27531745 Free PMC article.
-
Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A.Mol Cell Biol. 2001 Mar;21(6):1921-9. doi: 10.1128/MCB.21.6.1921-1929.2001. Mol Cell Biol. 2001. PMID: 11238928 Free PMC article.
-
A RSK(y) relationship with promiscuous PKA.Sci STKE. 2006 Aug 22;2006(349):pe32. doi: 10.1126/stke.3492006pe32. Sci STKE. 2006. PMID: 16926362 Review.
-
Regulation of MEK/ERK pathway output by subcellular localization of B-Raf.Biochem Soc Trans. 2012 Feb;40(1):67-72. doi: 10.1042/BST20110621. Biochem Soc Trans. 2012. PMID: 22260667 Review.
Cited by
-
cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression.J Biol Chem. 2010 May 7;285(19):14791-805. doi: 10.1074/jbc.M110.116905. Epub 2010 Feb 23. J Biol Chem. 2010. PMID: 20178980 Free PMC article.
-
Control of βAR- and N-methyl-D-aspartate (NMDA) Receptor-Dependent cAMP Dynamics in Hippocampal Neurons.PLoS Comput Biol. 2016 Feb 22;12(2):e1004735. doi: 10.1371/journal.pcbi.1004735. eCollection 2016 Feb. PLoS Comput Biol. 2016. PMID: 26901880 Free PMC article.
-
Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP.J Biol Chem. 2017 Jan 27;292(4):1449-1461. doi: 10.1074/jbc.M116.768986. Epub 2016 Dec 21. J Biol Chem. 2017. PMID: 28003362 Free PMC article.
-
Ras-mutant cancer cells display B-Raf binding to Ras that activates extracellular signal-regulated kinase and is inhibited by protein kinase A phosphorylation.J Biol Chem. 2013 Sep 20;288(38):27646-27657. doi: 10.1074/jbc.M113.463067. Epub 2013 Jul 26. J Biol Chem. 2013. PMID: 23893412 Free PMC article.
-
Trace amine-associated receptor 1 (TAAR1) promotes anti-diabetic signaling in insulin-secreting cells.J Biol Chem. 2019 Mar 22;294(12):4401-4411. doi: 10.1074/jbc.RA118.005464. Epub 2019 Jan 22. J Biol Chem. 2019. PMID: 30670596 Free PMC article.
References
-
- Ambrosini A, Tininini S, Barassi A, Racagni G, Sturani E, Zippel R. (2000) cAMP cascade leads to Ras activation in cortical neurons. Brain Res Mol Brain Res 75:54–60 - PubMed
-
- Boikos SA, Stratakis CA. (2007) Carney complex: the first 20 years. Curr Opin Oncol 19:24–29 - PubMed
-
- Cass LA, Meinkoth JL. (1998) Differential effects of cyclic adenosine 3′,5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology 139:1991–1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
