Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP
- PMID: 19718440
- PMCID: PMC2729930
- DOI: 10.1371/journal.pone.0006868
Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP
Abstract
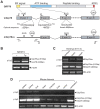
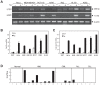
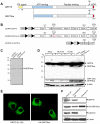
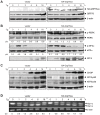
The unfolded protein response (UPR) is an evolutionarily conserved mechanism to allow cells to adapt to stress targeting the endoplasmic reticulum (ER). Induction of ER chaperone GRP78/BiP increases protein folding capacity; as such it represents a major survival arm of UPR. Considering the central importance of the UPR in regulating cell survival and death, evidence is emerging that cells evolve feedback regulatory pathways to modulate the key UPR executors, however, the precise mechanisms remain to be elucidated. Here, we report the fortuitous discovery of GRP78va, a novel isoform of GRP78 generated by alternative splicing (retention of intron 1) and alternative translation initiation. Bioinformatic and biochemical analyses revealed that expression of GRP78va is enhanced by ER stress and is notably elevated in human leukemic cells and leukemia patients. In contrast to the canonical GRP78 which is primarily an ER lumenal protein, GRP78va is devoid of the ER signaling peptide and is cytosolic. Through specific knockdown of endogenous GRP78va by siRNA without affecting canonical GRP78, we showed that GRP78va promotes cell survival under ER stress. We further demonstrated that GRP78va has the ability to regulate PERK signaling and that GRP78va is able to interact with and antagonize PERK inhibitor P58(IPK). Our study describes the discovery of GRP78va, a novel cytosolic isoform of GRP78/BiP, and the first characterization of the modulation of UPR signaling via alternative splicing of nuclear pre-mRNA. Our study further reveals a novel survival mechanism in leukemic cells and other cell types where GRP78va is expressed.
Conflict of interest statement
Figures

Similar articles
-
Measurement and modification of the expression level of the chaperone protein and signaling regulator GRP78/BiP in mammalian cells.Methods Enzymol. 2011;490:217-33. doi: 10.1016/B978-0-12-385114-7.00013-1. Methods Enzymol. 2011. PMID: 21266253
-
Mammalian ECD Protein Is a Novel Negative Regulator of the PERK Arm of the Unfolded Protein Response.Mol Cell Biol. 2017 Aug 28;37(18):e00030-17. doi: 10.1128/MCB.00030-17. Print 2017 Sep 15. Mol Cell Biol. 2017. PMID: 28652267 Free PMC article.
-
Inhibition of endoplasmic reticulum chaperone protein glucose-regulated protein 78 potentiates anti-angiogenic therapy in renal cell carcinoma through inactivation of the PERK/eIF2α pathway.Oncotarget. 2015 Oct 27;6(33):34818-30. doi: 10.18632/oncotarget.5397. Oncotarget. 2015. PMID: 26472187 Free PMC article.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
ER Stress and Unfolded Protein Response in Leukemia: Friend, Foe, or Both?Biomolecules. 2021 Jan 30;11(2):199. doi: 10.3390/biom11020199. Biomolecules. 2021. PMID: 33573353 Free PMC article. Review.
Cited by
-
The Gα-interacting vesicle-associated protein interacts with and promotes cell surface localization of GRP78 during endoplasmic reticulum stress.FEBS Lett. 2020 Mar;594(6):1088-1100. doi: 10.1002/1873-3468.13685. Epub 2019 Nov 30. FEBS Lett. 2020. PMID: 31736058 Free PMC article.
-
Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells.Cell Death Dis. 2010 Sep 9;1(9):e72. doi: 10.1038/cddis.2010.50. Cell Death Dis. 2010. PMID: 21364676 Free PMC article.
-
Proteostasis control by the unfolded protein response.Nat Cell Biol. 2015 Jul;17(7):829-38. doi: 10.1038/ncb3184. Nat Cell Biol. 2015. PMID: 26123108 Free PMC article. Review.
-
Protective unfolded protein response in human pancreatic beta cells transplanted into mice.PLoS One. 2010 Jun 18;5(6):e11211. doi: 10.1371/journal.pone.0011211. PLoS One. 2010. PMID: 20585452 Free PMC article.
-
Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential.Nat Rev Cancer. 2014 Apr;14(4):263-76. doi: 10.1038/nrc3701. Nat Rev Cancer. 2014. PMID: 24658275 Free PMC article. Review.
References
-
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. - PubMed
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. - PubMed
-
- Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–297. - PubMed
-
- Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

