Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes
- PMID: 19706553
- PMCID: PMC2728108
- DOI: 10.1073/pnas.0902809106
Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes
Abstract
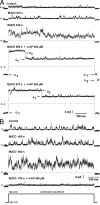
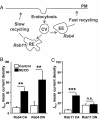
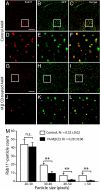
Cholesterol is an important determinant of cardiac electrical properties. However, underlying mechanisms are still poorly understood. Here, we examine the hypothesis that cholesterol modulates the turnover of voltage-gated potassium channels based on previous observations showing that depletion of membrane cholesterol increases the atrial repolarizing current I(Kur). Whole-cell currents and single-channel activity were recorded in rat adult atrial myocytes (AAM) or after transduction with hKv1.5-EGFP. Channel mobility and expression were studied using fluorescence recovery after photobleaching (FRAP) and 3-dimensional microscopy. In both native and transduced-AAMs, the cholesterol-depleting agent MbetaCD induced a delayed ( approximately 7 min) increase in I(Kur); the cholesterol donor LDL had an opposite effect. Single-channel recordings revealed an increased number of active Kv1.5 channels upon MbetaCD application. Whole-cell recordings indicated that this increase was not dependent on new synthesis but on trafficking of existing pools of intracellular channels whose exocytosis could be blocked by both N-ethylmaleimide and nonhydrolyzable GTP analogues. Rab11 was found to coimmunoprecipitate with hKv1.5-EGFP channels and transfection with Rab11 dominant negative (DN) but not Rab4 DN prevented the MbetaCD-induced I(Kur) increase. Three-dimensional microscopy showed a decrease in colocalization of Kv1.5 and Rab11 in MbetaCD-treated AAM. These results suggest that cholesterol regulates Kv1.5 channel expression by modulating its trafficking through the Rab11-associated recycling endosome. Therefore, this compartment provides a submembrane pool of channels readily available for recruitment into the sarcolemma of myocytes. This process could be a major mechanism for the tuning of cardiac electrical properties and might contribute to the understanding of cardiac effects of lipid-lowering drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
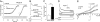


Similar articles
-
Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes.J Physiol. 2007 Aug 1;582(Pt 3):1205-17. doi: 10.1113/jphysiol.2007.134809. Epub 2007 May 24. J Physiol. 2007. PMID: 17525113 Free PMC article.
-
Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes.J Biol Chem. 2007 Oct 5;282(40):29612-20. doi: 10.1074/jbc.M704402200. Epub 2007 Aug 2. J Biol Chem. 2007. PMID: 17673464
-
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes.Circ Res. 2014 Mar 14;114(6):982-92. doi: 10.1161/CIRCRESAHA.114.302711. Epub 2014 Feb 7. Circ Res. 2014. PMID: 24508725 Free PMC article.
-
The anchoring protein SAP97 retains Kv1.5 channels in the plasma membrane of cardiac myocytes.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1851-61. doi: 10.1152/ajpheart.01045.2007. Epub 2008 Feb 1. Am J Physiol Heart Circ Physiol. 2008. PMID: 18245566
-
Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications.Cardiovasc Res. 2011 Mar 1;89(4):776-85. doi: 10.1093/cvr/cvq398. Epub 2010 Dec 15. Cardiovasc Res. 2011. PMID: 21159668 Review.
Cited by
-
Association between total cholesterol and all-cause mortality in oldest old: a national longitudinal study.Front Endocrinol (Lausanne). 2024 Jun 13;15:1405283. doi: 10.3389/fendo.2024.1405283. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38938514 Free PMC article.
-
The cellular pathways that maintain the quality control and transport of diverse potassium channels.Biochim Biophys Acta Gene Regul Mech. 2023 Mar;1866(1):194908. doi: 10.1016/j.bbagrm.2023.194908. Epub 2023 Jan 10. Biochim Biophys Acta Gene Regul Mech. 2023. PMID: 36638864 Free PMC article.
-
Lipid profile and incidence of atrial fibrillation: A prospective cohort study in China.Clin Cardiol. 2018 Mar;41(3):314-320. doi: 10.1002/clc.22864. Epub 2018 Mar 25. Clin Cardiol. 2018. PMID: 29575115 Free PMC article.
-
Association of Lipid-Related Genetic Variants with the Incidence of Atrial Fibrillation: The AFGen Consortium.PLoS One. 2016 Mar 21;11(3):e0151932. doi: 10.1371/journal.pone.0151932. eCollection 2016. PLoS One. 2016. PMID: 26999784 Free PMC article.
-
Association of hyperglycemia ratio and ventricular arrhythmia in critically ill patients admitted to the intensive care unit.BMC Cardiovasc Disord. 2023 Apr 28;23(1):215. doi: 10.1186/s12872-023-03208-9. BMC Cardiovasc Disord. 2023. PMID: 37118670 Free PMC article.
References
-
- Lundbaek JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996;35:3825–3830. - PubMed
-
- Oliver D, et al. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–270. - PubMed
-
- Savelieva I, Camm J. Statins and polyunsaturated fatty acids for treatment of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:30–41. - PubMed
-
- Tamargo J, et al. Lipid-lowering therapy with statins, a new approach to antiarrhythmic therapy. Pharmacol Ther. 2007;114:107–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

