Abnormal regulation of TSG101 in mice with spongiform neurodegeneration
- PMID: 19703557
- PMCID: PMC2755232
- DOI: 10.1016/j.bbadis.2009.08.009
Abnormal regulation of TSG101 in mice with spongiform neurodegeneration
Abstract
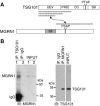
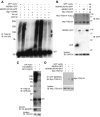
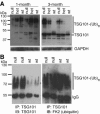
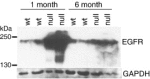
Spongiform neurodegeneration is characterized by the appearance of vacuoles throughout the central nervous system. It has many potential causes, but the underlying cellular mechanisms are not well understood. Mice lacking the E3 ubiquitin ligase Mahogunin Ring Finger-1 (MGRN1) develop age-dependent spongiform encephalopathy. We identified an interaction between a "PSAP" motif in MGRN1 and the ubiquitin E2 variant (UEV) domain of TSG101, a component of the endosomal sorting complex required for transport I (ESCRT-I), and demonstrate that MGRN1 multimonoubiquitinates TSG101. We examined the in vivo consequences of loss of MGRN1 on TSG101 expression and function in the mouse brain. The pattern of TSG101 ubiquitination differed in the brains of wild-type mice and Mgrn1 null mutant mice: at 1 month of age, null mutant mice had less ubiquitinated TSG101, while in adults, mutant mice had more ubiquitinated, insoluble TSG101 than wild-type mice. There was an associated increase in epidermal growth factor receptor (EGFR) levels in mutant brains. These results suggest that loss of MGRN1 promotes ubiquitination of TSG101 by other E3s and may prevent its disassociation from endosomal membranes or cause it to form insoluble aggregates. Our data implicate loss of normal TSG101 function in endo-lysosomal trafficking in the pathogenesis of spongiform neurodegeneration in Mgrn1 null mutant mice.
Figures


Similar articles
-
Oligodendroglial deletion of ESCRT-I component TSG101 causes spongiform encephalopathy.Biol Cell. 2016 Nov;108(11):324-337. doi: 10.1111/boc.201600014. Epub 2016 Aug 23. Biol Cell. 2016. PMID: 27406702
-
Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking.Mol Biol Cell. 2007 Apr;18(4):1129-42. doi: 10.1091/mbc.e06-09-0787. Epub 2007 Jan 17. Mol Biol Cell. 2007. PMID: 17229889 Free PMC article.
-
MGRN1-dependent pigment-type switching requires its ubiquitination activity but not its interaction with TSG101 or NEDD4.Pigment Cell Melanoma Res. 2013 Mar;26(2):263-8. doi: 10.1111/pcmr.12059. Epub 2013 Jan 8. Pigment Cell Melanoma Res. 2013. PMID: 23253940 Free PMC article.
-
Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking.Viruses. 2021 Jun 15;13(6):1147. doi: 10.3390/v13061147. Viruses. 2021. PMID: 34203832 Free PMC article. Review.
-
[The action of TSG101 on HIV-1 budding and related inhibitors].Yao Xue Xue Bao. 2008 Dec;43(12):1165-70. Yao Xue Xue Bao. 2008. PMID: 19244744 Review. Chinese.
Cited by
-
Chronic and age-dependent effects of the spongiform neurodegeneration-associated MGRN1 E3 ubiquitin ligase on mitochondrial homeostasis.Mamm Genome. 2019 Jun;30(5-6):151-165. doi: 10.1007/s00335-019-09802-7. Epub 2019 May 14. Mamm Genome. 2019. PMID: 31089807 Free PMC article.
-
The Multifaceted Roles of the Tumor Susceptibility Gene 101 (TSG101) in Normal Development and Disease.Cancers (Basel). 2020 Feb 14;12(2):450. doi: 10.3390/cancers12020450. Cancers (Basel). 2020. PMID: 32075127 Free PMC article. Review.
-
Functional conservation between mammalian MGRN1 and plant LOG2 ubiquitin ligases.FEBS Lett. 2013 Nov 1;587(21):3400-5. doi: 10.1016/j.febslet.2013.08.045. Epub 2013 Sep 10. FEBS Lett. 2013. PMID: 24036454 Free PMC article.
-
TSG101 Promotes the Proliferation, Migration, and Invasion of Human Glioma Cells by Regulating the AKT/GSK3β/β-Catenin and RhoC/Cofilin Pathways.Mol Neurobiol. 2021 May;58(5):2118-2132. doi: 10.1007/s12035-020-02231-7. Epub 2021 Jan 7. Mol Neurobiol. 2021. PMID: 33411238
-
Mahogunin Ring Finger 1 Is Required for Genomic Stability and Modulates the Malignant Phenotype of Melanoma Cells.Cancers (Basel). 2020 Oct 1;12(10):2840. doi: 10.3390/cancers12102840. Cancers (Basel). 2020. PMID: 33019669 Free PMC article.
References
-
- Everall I., Luthert P., Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J. Neuropathol. Exp. Neurol. 1993;52:561–566. - PubMed
-
- Georgsson G., Palsson P.A., Panitch H., Nathanson N., Petursson G. The ultrastructure of early visna lesions. Acta Neuropathol. (Berl). 1977;37:127–135. - PubMed
-
- Baslow M.H. Canavan's spongiform leukodystrophy: a clinical anatomy of a genetic metabolic CNS disease. J. Mol. Neurosci. 2000;15:61–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

