HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection
- PMID: 19702505
- PMCID: PMC2938771
- DOI: 10.1086/605648
HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection
Abstract
Background: Endemic transmission of measles continues in many countries that have a high human immunodeficiency virus (HIV) burden. The effects that HIV infection has on immune responses to measles and to measles vaccine can impact measles elimination efforts. Assays to measure antibody include the enzyme immunoassay (EIA), which measures immunoglobulin G (IgG) to all measles virus (MV) proteins, and the plaque reduction neutralization (PRN) assay, which measures antibody to the hemagglutinin and correlates with protection. Antibody avidity may affect neutralizing capacity.
Methods: HIV-infected and HIV-uninfected Zambian children were studied after measles vaccination (n=44) or MV infection (n=57). Laboratory or wild-type MV strains were used to infect Vero or Vero/signaling lymphocyte-activation molecule (SLAM) cells in PRN assays. IgG to MV was measured by EIA, and avidity was determined by ammonium thiocyanate dissociation.
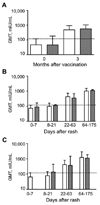
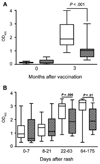
Results: HIV infection impaired EIA IgG responses after vaccination and measles but not PRN responses measured using laboratory-adapted MV. Avidity was lower among HIV-infected children 3 months after vaccination and 1 and 3 months after measles. Neutralization of wild-type MV infection of Vero/SLAM cells correlated with IgG avidity.
Conclusion: Lower antibody quality and quantity in HIV-infected children after measles vaccination raise challenges for assuring the long-term protection of these children. Antibody quality in children receiving antiretroviral therapy requires assessment.
Conflict of interest statement
Potential conflicts of interest: none reported.
Figures



Similar articles
-
Changes in measles serostatus among HIV-infected Zambian children initiating antiretroviral therapy before and after the 2010 measles outbreak and supplemental immunization activities.J Infect Dis. 2013 Dec 1;208(11):1747-55. doi: 10.1093/infdis/jit404. Epub 2013 Aug 2. J Infect Dis. 2013. PMID: 23911708 Free PMC article.
-
Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi.J Infect Dis. 2008 Nov 15;198(10):1457-65. doi: 10.1086/592756. J Infect Dis. 2008. PMID: 18828743 Clinical Trial.
-
Characterization of immune responses induced by intramuscular vaccination with DNA vaccines encoding measles virus hemagglutinin and/or fusion proteins.J Virol. 2005 Aug;79(15):9854-61. doi: 10.1128/JVI.79.15.9854-9861.2005. J Virol. 2005. PMID: 16014946 Free PMC article.
-
Measles Immunity at 4.5 Years of Age Following Vaccination at 9 and 15-18 Months of Age Among Human Immunodeficiency Virus (HIV)-infected, HIV-exposed-uninfected, and HIV-unexposed Children.Clin Infect Dis. 2019 Aug 1;69(4):687-696. doi: 10.1093/cid/ciy964. Clin Infect Dis. 2019. PMID: 30418528 Free PMC article. Clinical Trial.
-
Antiviral Functions of Human Immunodeficiency Virus Type 1 (HIV-1)-Specific IgG Antibodies: Effects of Antiretroviral Therapy and Implications for Therapeutic HIV-1 Vaccine Design.Front Immunol. 2017 Jul 4;8:780. doi: 10.3389/fimmu.2017.00780. eCollection 2017. Front Immunol. 2017. PMID: 28725225 Free PMC article. Review.
Cited by
-
Control of Viremia Enables Acquisition of Resting Memory B Cells with Age and Normalization of Activated B Cell Phenotypes in HIV-Infected Children.J Immunol. 2015 Aug 1;195(3):1082-91. doi: 10.4049/jimmunol.1500491. Epub 2015 Jun 26. J Immunol. 2015. PMID: 26116511 Free PMC article.
-
Characterising antibody avidity in individuals of varied Mycobacterium tuberculosis infection status using surface plasmon resonance.PLoS One. 2018 Oct 12;13(10):e0205102. doi: 10.1371/journal.pone.0205102. eCollection 2018. PLoS One. 2018. PMID: 30312318 Free PMC article.
-
Safety and Immunogenicity of Measles Vaccination in HIV-Infected and HIV-Exposed Uninfected Children: A Systematic Review and Meta-Analysis.EClinicalMedicine. 2018 Jul 2;1:28-42. doi: 10.1016/j.eclinm.2018.06.002. eCollection 2018 Jul. EClinicalMedicine. 2018. PMID: 31193646 Free PMC article.
-
Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings.Clin Vaccine Immunol. 2012 Nov;19(11):1810-7. doi: 10.1128/CVI.00406-12. Epub 2012 Sep 12. Clin Vaccine Immunol. 2012. PMID: 22971778 Free PMC article.
-
Immunologic basis for revaccination of HIV-infected children receiving HAART.Future Virol. 2011 Jan 1;6(1):59-71. doi: 10.2217/fvl.10.75. Future Virol. 2011. PMID: 21339832 Free PMC article.
References
-
- Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet. 2007;369:191–200. - PubMed
-
- Cutts FT, Henao-Restrepo A, Olive JM. Measles elimination: progress and challenges. Vaccine. 1999;17 Suppl 3:S47–S52. - PubMed
-
- Moss WJ, Cutts F, Griffin DE. Implications of the HIV epidemic for control and eradication of measles. Clin Infect Dis. 1999;29:106–112. - PubMed
-
- Scott S, Moss WJ, Cousens S, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–1424. - PubMed
-
- Centers for Disease Control. Measles in HIV-infected children, United States. MMWR Morb Mortal Wkly Rep. 1988;37:183–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

