CpG methylation controls reactivation of HIV from latency
- PMID: 19696893
- PMCID: PMC2722084
- DOI: 10.1371/journal.ppat.1000554
CpG methylation controls reactivation of HIV from latency
Abstract
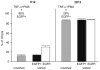
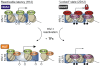
DNA methylation of retroviral promoters and enhancers localized in the provirus 5' long terminal repeat (LTR) is considered to be a mechanism of transcriptional suppression that allows retroviruses to evade host immune responses and antiretroviral drugs. However, the role of DNA methylation in the control of HIV-1 latency has never been unambiguously demonstrated, in contrast to the apparent importance of transcriptional interference and chromatin structure, and has never been studied in HIV-1-infected patients. Here, we show in an in vitro model of reactivable latency and in a latent reservoir of HIV-1-infected patients that CpG methylation of the HIV-1 5' LTR is an additional epigenetic restriction mechanism, which controls resistance of latent HIV-1 to reactivation signals and thus determines the stability of the HIV-1 latency. CpG methylation acts as a late event during establishment of HIV-1 latency and is not required for the initial provirus silencing. Indeed, the latent reservoir of some aviremic patients contained high proportions of the non-methylated 5' LTR. The latency controlled solely by transcriptional interference and by chromatin-dependent mechanisms in the absence of significant promoter DNA methylation tends to be leaky and easily reactivable. In the latent reservoir of HIV-1-infected individuals without detectable plasma viremia, we found HIV-1 promoters and enhancers to be hypermethylated and resistant to reactivation, as opposed to the hypomethylated 5' LTR in viremic patients. However, even dense methylation of the HIV-1 5'LTR did not confer complete resistance to reactivation of latent HIV-1 with some histone deacetylase inhibitors, protein kinase C agonists, TNF-alpha, and their combinations with 5-aza-2deoxycytidine: the densely methylated HIV-1 promoter was most efficiently reactivated in virtual absence of T cell activation by suberoylanilide hydroxamic acid. Tight but incomplete control of HIV-1 latency by CpG methylation might have important implications for strategies aimed at eradicating HIV-1 infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Development of 5' LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals.Clin Epigenetics. 2016 Feb 19;8:19. doi: 10.1186/s13148-016-0185-6. eCollection 2016. Clin Epigenetics. 2016. PMID: 26900410 Free PMC article.
-
5' long terminal repeat (LTR)-selective methylation of latently infected HIV-1 provirus that is demethylated by reactivation signals.Retrovirology. 2006 Oct 12;3:69. doi: 10.1186/1742-4690-3-69. Retrovirology. 2006. PMID: 17034647 Free PMC article.
-
RNA-induced epigenetic silencing inhibits HIV-1 reactivation from latency.Retrovirology. 2018 Oct 4;15(1):67. doi: 10.1186/s12977-018-0451-0. Retrovirology. 2018. PMID: 30286764 Free PMC article.
-
HIV-Induced Epigenetic Alterations in Host Cells.Adv Exp Med Biol. 2016;879:27-38. doi: 10.1007/978-3-319-24738-0_2. Adv Exp Med Biol. 2016. PMID: 26659262 Review.
-
In vivo, in vitro, and in silico analysis of methylation of the HIV-1 provirus.Methods. 2011 Jan;53(1):47-53. doi: 10.1016/j.ymeth.2010.05.009. Epub 2010 Jun 2. Methods. 2011. PMID: 20670606 Free PMC article. Review.
Cited by
-
Current views on HIV-1 latency, persistence, and cure.Folia Microbiol (Praha). 2017 Jan;62(1):73-87. doi: 10.1007/s12223-016-0474-7. Epub 2016 Oct 5. Folia Microbiol (Praha). 2017. PMID: 27709447 Review.
-
Novel role of UHRF1 in the epigenetic repression of the latent HIV-1.EBioMedicine. 2022 May;79:103985. doi: 10.1016/j.ebiom.2022.103985. Epub 2022 Apr 14. EBioMedicine. 2022. PMID: 35429693 Free PMC article.
-
The lysine methyltransferase SMYD5 amplifies HIV-1 transcription and is post-transcriptionally upregulated by Tat and USP11.Cell Rep. 2023 Mar 28;42(3):112234. doi: 10.1016/j.celrep.2023.112234. Epub 2023 Mar 9. Cell Rep. 2023. PMID: 36897778 Free PMC article.
-
HIV cure strategies: which ones are appropriate for Africa?Cell Mol Life Sci. 2022 Jul 6;79(8):400. doi: 10.1007/s00018-022-04421-z. Cell Mol Life Sci. 2022. PMID: 35794316 Free PMC article. Review.
-
Insights Into Persistent HIV-1 Infection and Functional Cure: Novel Capabilities and Strategies.Front Microbiol. 2022 Apr 27;13:862270. doi: 10.3389/fmicb.2022.862270. eCollection 2022. Front Microbiol. 2022. PMID: 35572626 Free PMC article. Review.
References
-
- Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. - PubMed
-
- Ramratnam B, Mittler JE, Zhang L, Boden D, Hurley A, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–85. - PubMed
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

