Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src
- PMID: 19692574
- PMCID: PMC2762143
- DOI: 10.1091/mbc.e08-11-1158
Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src
Abstract
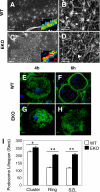
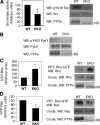
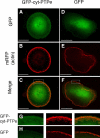
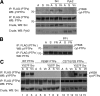
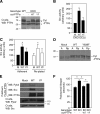
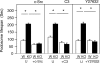
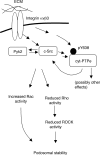
The nonreceptor isoform of tyrosine phosphatase epsilon (cyt-PTPe) supports osteoclast adhesion and activity in vivo, leading to increased bone mass in female mice lacking PTPe (EKO mice). The structure and organization of the podosomal adhesion structures of EKO osteoclasts are abnormal; the molecular mechanism behind this is unknown. We show here that EKO podosomes are disorganized, unusually stable, and reorganize poorly in response to physical contact. Phosphorylation and activities of Src, Pyk2, and Rac are decreased and Rho activity is increased in EKO osteoclasts, suggesting that integrin signaling is defective in these cells. Integrin activation regulates cyt-PTPe by inducing Src-dependent phosphorylation of cyt-PTPe at Y638. This phosphorylation event is crucial because wild-type-but not Y638F-cyt-PTPe binds and further activates Src and restores normal stability to podosomes in EKO osteoclasts. Increasing Src activity or inhibiting Rho or its downstream effector Rho kinase in EKO osteoclasts rescues their podosomal stability phenotype, indicating that cyt-PTPe affects podosome stability by functioning upstream of these molecules. We conclude that cyt-PTPe participates in a feedback loop that ensures proper Src activation downstream of integrins, thus linking integrin signaling with Src activation and accurate organization and stability of podosomes in osteoclasts.
Figures

Similar articles
-
Protein tyrosine phosphatases ε and α perform nonredundant roles in osteoclasts.Mol Biol Cell. 2014 Jun;25(11):1808-18. doi: 10.1091/mbc.E14-03-0788. Epub 2014 Apr 2. Mol Biol Cell. 2014. PMID: 24694598 Free PMC article.
-
Adaptor protein GRB2 promotes Src tyrosine kinase activation and podosomal organization by protein-tyrosine phosphatase ϵ in osteoclasts.J Biol Chem. 2014 Dec 26;289(52):36048-58. doi: 10.1074/jbc.M114.603548. Epub 2014 Nov 7. J Biol Chem. 2014. PMID: 25381250 Free PMC article.
-
Integrin-mediated signaling in the regulation of osteoclast adhesion and activation.Front Biosci. 1998 Aug 1;3:d757-68. doi: 10.2741/A319. Front Biosci. 1998. PMID: 9682033 Review.
-
Abnormal localisation and hyperclustering of (alpha)(V)(beta)(3) integrins and associated proteins in Src-deficient or tyrphostin A9-treated osteoclasts.J Cell Sci. 2001 Jan;114(Pt 1):149-160. doi: 10.1242/jcs.114.1.149. J Cell Sci. 2001. PMID: 11112699
-
Podosome and sealing zone: specificity of the osteoclast model.Eur J Cell Biol. 2006 Apr;85(3-4):195-202. doi: 10.1016/j.ejcb.2005.09.008. Epub 2005 Oct 24. Eur J Cell Biol. 2006. PMID: 16546562 Review.
Cited by
-
Podosome organization drives osteoclast-mediated bone resorption.Cell Adh Migr. 2014;8(3):191-204. doi: 10.4161/cam.27840. Cell Adh Migr. 2014. PMID: 24714644 Free PMC article. Review.
-
Protein tyrosine phosphatases ε and α perform nonredundant roles in osteoclasts.Mol Biol Cell. 2014 Jun;25(11):1808-18. doi: 10.1091/mbc.E14-03-0788. Epub 2014 Apr 2. Mol Biol Cell. 2014. PMID: 24694598 Free PMC article.
-
Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation.J Cell Mol Med. 2020 Mar;24(6):3271-3281. doi: 10.1111/jcmm.15052. Epub 2020 Feb 11. J Cell Mol Med. 2020. PMID: 32045092 Free PMC article. Review.
-
The Configuration of GRB2 in Protein Interaction and Signal Transduction.Biomolecules. 2024 Feb 22;14(3):259. doi: 10.3390/biom14030259. Biomolecules. 2024. PMID: 38540680 Free PMC article. Review.
-
Gα13 negatively controls osteoclastogenesis through inhibition of the Akt-GSK3β-NFATc1 signalling pathway.Nat Commun. 2017 Jan 19;8:13700. doi: 10.1038/ncomms13700. Nat Commun. 2017. PMID: 28102206 Free PMC article.
References
-
- Amoui M., Sheng M. H., Chen S. T., Baylink D. J., Lau K. H. A transmembrane osteoclastic protein-tyrosine phosphatase regulates osteoclast activity in part by promoting osteoclast survival through c-Src-dependent activation of NFkappaB and JNK2. Arch. Biochem. Biophys. 2007;463:47–59. - PubMed
-
- Aoki K., Didomenico E., Sims N. A., Mukhopadhyay K., Neff L., Houghton A., Amling M., Levy J. B., Horne W. C., Baron R. The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in me(v)/me(v) mutant mice. Bone. 1999;25:261–267. - PubMed
-
- Arthur W. T., Petch L. A., Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 2000;10:719–722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

