A central role for monocytes in Toll-like receptor-mediated activation of the vasculature
- PMID: 19689736
- PMCID: PMC2747139
- DOI: 10.1111/j.1365-2567.2009.03071.x
A central role for monocytes in Toll-like receptor-mediated activation of the vasculature
Abstract
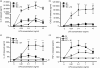
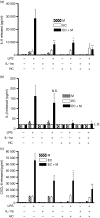
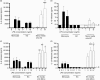
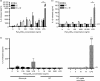
There is increasing evidence that activation of inflammatory responses in a variety of tissues is mediated co-operatively by the actions of more than one cell type. In particular, the monocyte has been implicated as a potentially important cell in the initiation of inflammatory responses to Toll-like receptor (TLR)-activating signals. To determine the potential for monocyte-regulated activation of tissue cells to underpin inflammatory responses in the vasculature, we established cocultures of primary human endothelial cells and monocytes and dissected the inflammatory responses of these systems following activation with TLR agonists. We observed that effective activation of inflammatory responses required bidirectional signalling between the monocyte and the tissue cell. Activation of cocultures was dependent on interleukin-1 (IL-1). Although monocyte-mediated IL-1beta production was crucial to the activation of cocultures, TLR specificity to these responses was also provided by the endothelial cells, which served to regulate the signalling of the monocytes. TLR4-induced IL-1beta production by monocytes was increased by TLR4-dependent endothelial activation in coculture, and was associated with increased monocyte CD14 expression. Activation of this inflammatory network also supported the potential for downstream monocyte-dependent T helper type 17 activation. These data define co-operative networks regulating inflammatory responses to TLR agonists, identify points amenable to targeting for the amelioration of vascular inflammation, and offer the potential to modify atherosclerotic plaque instability after a severe infection.
Figures
Similar articles
-
Differential and cell-type specific regulation of responses to Toll-like receptor agonists by ISO-1.Immunology. 2008 Sep;125(1):101-10. doi: 10.1111/j.1365-2567.2008.02825.x. Epub 2008 Mar 18. Immunology. 2008. PMID: 18355244 Free PMC article.
-
Toll-like receptors elicit different recruitment kinetics of monocytes and neutrophils in mouse acute inflammation.Eur J Immunol. 2017 Jun;47(6):1002-1008. doi: 10.1002/eji.201746983. Epub 2017 Apr 18. Eur J Immunol. 2017. PMID: 28299776
-
Female sex hormones modulate Porphyromonas gingivalis lipopolysaccharide-induced Toll-like receptor signaling in primary human monocytes.J Periodontal Res. 2016 Jun;51(3):395-406. doi: 10.1111/jre.12320. Epub 2015 Sep 14. J Periodontal Res. 2016. PMID: 26364725
-
Immunological and inflammatory functions of the interleukin-1 family.Annu Rev Immunol. 2009;27:519-50. doi: 10.1146/annurev.immunol.021908.132612. Annu Rev Immunol. 2009. PMID: 19302047 Review.
-
Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis.Ann Periodontol. 2002 Dec;7(1):72-8. doi: 10.1902/annals.2002.7.1.72. Ann Periodontol. 2002. PMID: 16013219 Review.
Cited by
-
Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium.J Biol Chem. 2015 Oct 2;290(40):24067-78. doi: 10.1074/jbc.M115.659029. Epub 2015 Aug 12. J Biol Chem. 2015. PMID: 26269588 Free PMC article.
-
Interleukin-1β regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intercellular cooperation between pulmonary epithelial cells and macrophages.Infect Immun. 2012 Mar;80(3):1140-9. doi: 10.1128/IAI.05697-11. Epub 2011 Dec 12. Infect Immun. 2012. PMID: 22158745 Free PMC article.
-
Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson's disease.Int J Neuropsychopharmacol. 2014 Dec 7;18(6):pyu103. doi: 10.1093/ijnp/pyu103. Int J Neuropsychopharmacol. 2014. PMID: 25522431 Free PMC article.
-
Pellino-1 selectively regulates epithelial cell responses to rhinovirus.J Virol. 2012 Jun;86(12):6595-604. doi: 10.1128/JVI.06755-11. Epub 2012 Apr 18. J Virol. 2012. PMID: 22514342 Free PMC article.
-
Assessing the immunopotency of Toll-like receptor agonists in an in vitro tissue-engineered immunological model.Immunology. 2010 Jul;130(3):374-87. doi: 10.1111/j.1365-2567.2009.03237.x. Epub 2010 Mar 17. Immunology. 2010. PMID: 20331478 Free PMC article.
References
-
- Morris GE, Parker LC, Ward JR, et al. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006;20:2153–5. - PubMed
-
- Morris GE, Whyte MKB, Martin GF, Jose PJ, Dower SK, Sabroe I. Agonists of Toll-like receptors 2 and 4 activate airway smooth muscle via mononuclear leukocytes. Am J Respir Crit Care Med. 2005;171:814–22. - PubMed
-
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

