Gut commensal bacteria direct a protective immune response against Toxoplasma gondii
- PMID: 19683684
- PMCID: PMC2746820
- DOI: 10.1016/j.chom.2009.06.005
Gut commensal bacteria direct a protective immune response against Toxoplasma gondii
Abstract
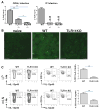
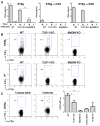
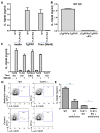
Toxoplasma gondii is a universally distributed pathogen that infects over one billion people worldwide. Host resistance to this protozoan parasite depends on a Th1 immune response with potent production of the cytokines interleukin-12 and interferon gamma. Although Toll-like receptor 11 (TLR11) plays a major role in controlling Th1 immunity to this pathogen in mice, this innate immune receptor is nonfunctional in humans, and the mechanisms of TLR11-independent sensing of T. gondii remain elusive. Here, we show that oral infection by T. gondii triggers a TLR11-independent but MyD88-dependent Th1 response that is impaired in TLR2xTLR4 double knockout and TLR9 single knockout mice. These mucosal innate and adaptive immune responses to T. gondii rely on the indirect stimulation of dendritic cells by normal gut microflora. Thus, our results reveal that gut commensal bacteria can serve as molecular adjuvants during parasitic infection, providing indirect immunostimulation that protects against T. gondii in the absence of TLR11.
Figures

Comment in
-
A gut feeling for microbes: getting it going between a parasite and its host.Cell Host Microbe. 2009 Aug 20;6(2):104-6. doi: 10.1016/j.chom.2009.07.009. Cell Host Microbe. 2009. PMID: 19683676 Review.
Similar articles
-
TLR11-independent inflammasome activation is critical for CD4+ T cell-derived IFN-γ production and host resistance to Toxoplasma gondii.PLoS Pathog. 2019 Jun 13;15(6):e1007872. doi: 10.1371/journal.ppat.1007872. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31194844 Free PMC article.
-
Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells.J Immunol. 2002 Jun 15;168(12):5997-6001. doi: 10.4049/jimmunol.168.12.5997. J Immunol. 2002. PMID: 12055206
-
Innate responses to Toxoplasma gondii in mice and humans.Trends Parasitol. 2011 Sep;27(9):388-93. doi: 10.1016/j.pt.2011.03.009. Trends Parasitol. 2011. PMID: 21550851 Free PMC article. Review.
-
TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii.J Immunol. 2006 Jun 15;176(12):7589-97. doi: 10.4049/jimmunol.176.12.7589. J Immunol. 2006. PMID: 16751405
-
A gut feeling for microbes: getting it going between a parasite and its host.Cell Host Microbe. 2009 Aug 20;6(2):104-6. doi: 10.1016/j.chom.2009.07.009. Cell Host Microbe. 2009. PMID: 19683676 Review.
Cited by
-
Toxoplasma-induced behavior changes - is microbial dysbiosis the missing link?Front Cell Infect Microbiol. 2024 Sep 30;14:1415079. doi: 10.3389/fcimb.2024.1415079. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39403206 Free PMC article. Review.
-
New advances in immune mechanism and treatment during ocular toxoplasmosis.Front Immunol. 2024 May 10;15:1403025. doi: 10.3389/fimmu.2024.1403025. eCollection 2024. Front Immunol. 2024. PMID: 38799473 Free PMC article. Review.
-
iNOS is necessary for GBP-mediated T. gondii clearance in murine macrophages via vacuole nitration and intravacuolar network collapse.Nat Commun. 2024 Mar 27;15(1):2698. doi: 10.1038/s41467-024-46790-y. Nat Commun. 2024. PMID: 38538595 Free PMC article.
-
Moniezia benedeni drives CD3+ T cells residence in the sheep intestinal mucosal effector sites.Front Vet Sci. 2024 Feb 2;11:1342169. doi: 10.3389/fvets.2024.1342169. eCollection 2024. Front Vet Sci. 2024. PMID: 38371601 Free PMC article.
-
The intersection of host in vivo metabolism and immune responses to infection with kinetoplastid and apicomplexan parasites.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0016422. doi: 10.1128/mmbr.00164-22. Epub 2024 Feb 1. Microbiol Mol Biol Rev. 2024. PMID: 38299836 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

