Hax1 lacks BH modules and is peripherally associated to heavy membranes: implications for Omi/HtrA2 and PARL activity in the regulation of mitochondrial stress and apoptosis
- PMID: 19680265
- PMCID: PMC4300852
- DOI: 10.1038/cdd.2009.110
Hax1 lacks BH modules and is peripherally associated to heavy membranes: implications for Omi/HtrA2 and PARL activity in the regulation of mitochondrial stress and apoptosis
Abstract
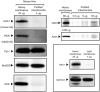
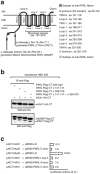
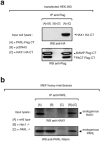
Hax1 has an important role in immunodeficiency syndromes and apoptosis. A recent report (Chao et al., Nature, 2008) proposed that the Bcl-2-family-related protein, Hax1, suppresses apoptosis in lymphocytes and neurons through a mechanism that involves its association to the inner mitochondrial membrane rhomboid protease PARL, to proteolytically activate the serine protease Omi/HtrA2 and eliminate active Bax. This model implies that the control of cell-type sensitivity to pro-apoptotic stimuli is governed by the PARL/Hax1 complex in the mitochondria intermembrane space and, more generally, that Bcl-2-family-related proteins can control mitochondrial outer-membrane permeabilization from inside the mitochondrion. Further, it defines a novel, anti-apoptotic Opa1-independent pathway for PARL. In this study, we present evidence that, in vivo, the activity of Hax1 cannot be mechanistically coupled to PARL because the two proteins are confined in distinct cellular compartments and their interaction in vitro is an artifact. We also show by sequence analysis and secondary structure prediction that Hax1 is extremely unlikely to be a Bcl-2-family-related protein because it lacks Bcl-2 homology modules. These results indicate a different function and mechanism of Hax1 in apoptosis and re-opens the question of whether mammalian PARL, in addition to apoptosis, regulates mitochondrial stress response through Omi/HtrA2 processing.
Figures



Similar articles
-
MLF1 is a proapoptotic antagonist of HOP complex-mediated survival.Biochim Biophys Acta Mol Cell Res. 2017 Apr;1864(4):719-727. doi: 10.1016/j.bbamcr.2017.01.016. Epub 2017 Jan 27. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28137643
-
Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons.Nature. 2008 Mar 6;452(7183):98-102. doi: 10.1038/nature06604. Epub 2008 Feb 20. Nature. 2008. PMID: 18288109
-
The role of PARL and HtrA2 in striatal neuronal injury after transient global cerebral ischemia.J Cereb Blood Flow Metab. 2013 Nov;33(11):1658-65. doi: 10.1038/jcbfm.2013.139. Epub 2013 Aug 7. J Cereb Blood Flow Metab. 2013. PMID: 23921894 Free PMC article.
-
A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis.Cell Death Differ. 2007 Jul;14(7):1275-84. doi: 10.1038/sj.cdd.4402145. Epub 2007 Apr 20. Cell Death Differ. 2007. PMID: 17464328 Review.
-
Progress in research on the role of Omi/HtrA2 in neurological diseases.Rev Neurosci. 2019 Apr 24;30(3):279-287. doi: 10.1515/revneuro-2018-0004. Rev Neurosci. 2019. PMID: 30205651 Review.
Cited by
-
Grb7 and Hax1 may colocalize partially to mitochondria in EGF-treated SKBR3 cells and their interaction can affect Caspase3 cleavage of Hax1.J Mol Recognit. 2016 Jul;29(7):318-33. doi: 10.1002/jmr.2533. Epub 2016 Feb 12. J Mol Recognit. 2016. PMID: 26869103 Free PMC article.
-
Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates.Mol Cell. 2009 Dec 25;36(6):1048-59. doi: 10.1016/j.molcel.2009.11.006. Mol Cell. 2009. PMID: 20064469 Free PMC article.
-
Anti-apoptotic HAX-1 suppresses cell apoptosis by promoting c-Abl kinase-involved ROS clearance.Cell Death Dis. 2022 Apr 4;13(4):298. doi: 10.1038/s41419-022-04748-2. Cell Death Dis. 2022. PMID: 35379774 Free PMC article.
-
Omi inhibition ameliorates neuron apoptosis and neurological deficit after subarachnoid hemorrhage in rats.Genes Genomics. 2021 Dec;43(12):1423-1432. doi: 10.1007/s13258-021-01176-y. Epub 2021 Oct 22. Genes Genomics. 2021. PMID: 34677809 Free PMC article.
-
HAX1 regulates E3 ubiquitin ligase activity of cIAPs by promoting their dimerization.Oncotarget. 2014 Oct 30;5(20):10084-99. doi: 10.18632/oncotarget.2459. Oncotarget. 2014. PMID: 25275296 Free PMC article.
References
-
- Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–60. - PubMed
-
- Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci. 2001;26:112–7. - PubMed
-
- Martinou JC, Youle RJ. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ. 2006;13:1291–5. - PubMed
-
- Jourdain A, Martinou JC. Mitochondrial outer-membrane permeabilization and remodelling in apoptosis. Int J Biochem Cell Biol. 2009 - PubMed
-
- Scorrano L. Opening the doors to cytochrome c: Changes in mitochondrial shape and apoptosis. Int J Biochem Cell Biol. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

