Postsynaptic development of the neuromuscular junction in mice lacking the gamma-subunit of muscle nicotinic acetylcholine receptor
- PMID: 19672725
- PMCID: PMC2819111
- DOI: 10.1007/s12031-009-9248-x
Postsynaptic development of the neuromuscular junction in mice lacking the gamma-subunit of muscle nicotinic acetylcholine receptor
Abstract
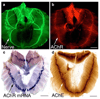
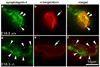
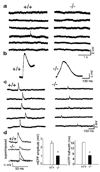
The mammalian muscle nicotinic acetylcholine receptor (AChR) is composed of five membrane-spanning subunits and its composition differs between embryonic and adult muscles. In embryonic muscles, it is composed of two alpha-, one beta-, one delta-, and one gamma-subunit; the gamma-subunit is later replaced by the epsilon-subunit during postnatal development. This unique temporal expression pattern of the gamma-subunit suggests it may play specific roles in embryonic muscles. To address this issue, we examined the formation and function of the neuromuscular junction in mouse embryos deficient in the gamma-subunit. At embryonic day 15.5, AChR clusters were absent and the spontaneous miniature endplate potentials were undetectable in the mutant muscles. However, electrical stimulation of the nerves triggered muscle contraction and elicited postsynaptic endplate potential (EPP) in the mutant muscles, although the magnitude of the muscle contraction and the amplitudes of the EPPs were smaller in the mutant compared to the wild-type muscles. Reintroducing a wild-type gamma-subunit into the mutant myotubes restored the formation of AChR clusters in vitro. Together, these results have demonstrated that functional AChRs were present in the mutant muscle membrane, but at reduced levels. Thus, in the absence of the gamma-subunit, a combination of alpha, beta, and delta subunits may assemble into functional receptors in vivo. These results also suggest that the gamma-subunit maybe involved in interacting with rapsyn, a cytoplasmic protein required for AChR clustering.
Figures

Similar articles
-
Aberrant development of motor axons and neuromuscular synapses in MyoD-null mice.J Neurosci. 2003 Jun 15;23(12):5161-9. doi: 10.1523/JNEUROSCI.23-12-05161.2003. J Neurosci. 2003. PMID: 12832540 Free PMC article.
-
Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse.Dev Neurobiol. 2008 Aug;68(9):1153-69. doi: 10.1002/dneu.20654. Dev Neurobiol. 2008. PMID: 18506821
-
Developmental increase in the amount of rapsyn per acetylcholine receptor promotes postsynaptic receptor packing and stability.Dev Biol. 2007 May 1;305(1):262-75. doi: 10.1016/j.ydbio.2007.02.008. Epub 2007 Feb 16. Dev Biol. 2007. PMID: 17362913
-
Congenital myasthenic syndromes: multiple molecular targets at the neuromuscular junction.Ann N Y Acad Sci. 2003 Sep;998:138-60. doi: 10.1196/annals.1254.016. Ann N Y Acad Sci. 2003. PMID: 14592871 Review.
-
Development of the neuromuscular junction.Cell Tissue Res. 2006 Nov;326(2):263-71. doi: 10.1007/s00441-006-0237-x. Epub 2006 Jul 4. Cell Tissue Res. 2006. PMID: 16819627 Review.
Cited by
-
Modification of Neuromuscular Junction Protein Expression by Exercise and Doxorubicin.Med Sci Sports Exerc. 2020 Jul;52(7):1477-1484. doi: 10.1249/MSS.0000000000002286. Med Sci Sports Exerc. 2020. PMID: 31985575 Free PMC article.
-
Muscle Yap Is a Regulator of Neuromuscular Junction Formation and Regeneration.J Neurosci. 2017 Mar 29;37(13):3465-3477. doi: 10.1523/JNEUROSCI.2934-16.2017. Epub 2017 Feb 17. J Neurosci. 2017. PMID: 28213440 Free PMC article.
-
Neuromotor synapses in Escobar syndrome.Am J Med Genet A. 2013 Dec;161A(12):3042-8. doi: 10.1002/ajmg.a.36154. Epub 2013 Aug 16. Am J Med Genet A. 2013. PMID: 24038971 Free PMC article.
-
VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions.Skelet Muscle. 2016 Oct 5;6:31. doi: 10.1186/s13395-016-0105-7. eCollection 2016. Skelet Muscle. 2016. PMID: 27713817 Free PMC article.
-
Ablation of Lrp4 in Schwann Cells Promotes Peripheral Nerve Regeneration in Mice.Biology (Basel). 2021 May 21;10(6):452. doi: 10.3390/biology10060452. Biology (Basel). 2021. PMID: 34063992 Free PMC article.
References
-
- Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. Journal Comparative Neurology. 1997a;382:469–479. - PubMed
-
- Allan DW, Greer JJ. Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. Journal Comparative Neurology. 1997b;382:459–468. - PubMed
-
- Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Current Opinion in Neurobiology. 2002;12:100–103. - PubMed
-
- Changeux JP, Devillers-Thiery A, Galzi JL, Revah F. The acetylcholine receptor: A model of an allosteric membrane protein mediating intercellular communication; Ciba Foundation Symposium; 1992. pp. 66–89. discussion 87–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

