Endoplasmic reticulum stress in beta-cells and development of diabetes
- PMID: 19665428
- PMCID: PMC2787771
- DOI: 10.1016/j.coph.2009.07.003
Endoplasmic reticulum stress in beta-cells and development of diabetes
Abstract
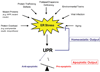
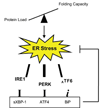
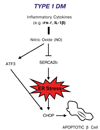
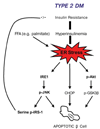
The endoplasmic reticulum (ER) is a cellular compartment responsible for multiple important cellular functions including the biosynthesis and folding of newly synthesized proteins destined for secretion, such as insulin. A myriad of pathological and physiological factors perturb ER function and cause dysregulation of ER homeostasis, leading to ER stress. ER stress elicits a signaling cascade to mitigate stress, the unfolded protein response (UPR). As long as the UPR can relieve stress, cells can produce the proper amount of proteins and maintain ER homeostasis. If the UPR, however, fails to maintain ER homeostasis, cells will undergo apoptosis. Activation of the UPR is critical to the survival of insulin-producing pancreatic beta-cells with high secretory protein production. Any disruption of ER homeostasis in beta-cells can lead to cell death and contribute to the pathogenesis of diabetes. There are several models of ER-stress-mediated diabetes. In this review, we outline the underlying molecular mechanisms of ER-stress-mediated beta-cell dysfunction and death during the progression of diabetes.
Figures
Similar articles
-
Stress hypERactivation in the β-cell.Islets. 2010 Jan-Feb;2(1):1-9. doi: 10.4161/isl.2.1.10456. Islets. 2010. PMID: 21099287 Review.
-
Guards and culprits in the endoplasmic reticulum: glucolipotoxicity and β-cell failure in type II diabetes.Exp Diabetes Res. 2012;2012:639762. doi: 10.1155/2012/639762. Epub 2011 Oct 1. Exp Diabetes Res. 2012. PMID: 21977023 Free PMC article. Review.
-
Endoplasmic reticulum stress and pancreatic β-cell death.Trends Endocrinol Metab. 2011 Jul;22(7):266-74. doi: 10.1016/j.tem.2011.02.008. Epub 2011 Mar 31. Trends Endocrinol Metab. 2011. PMID: 21458293 Free PMC article. Review.
-
ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes.Ups J Med Sci. 2016 May;121(2):133-9. doi: 10.3109/03009734.2015.1135217. Epub 2016 Feb 22. Ups J Med Sci. 2016. PMID: 26899404 Free PMC article. Review.
-
Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes.Mol Metab. 2019 Sep;27S(Suppl):S60-S68. doi: 10.1016/j.molmet.2019.06.012. Mol Metab. 2019. PMID: 31500832 Free PMC article. Review.
Cited by
-
A Quantitative Analysis of Cellular Lipid Compositions During Acute Proteotoxic ER Stress Reveals Specificity in the Production of Asymmetric Lipids.Front Cell Dev Biol. 2020 Aug 4;8:756. doi: 10.3389/fcell.2020.00756. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850859 Free PMC article.
-
Role of Endocrine-Disrupting Engineered Nanomaterials in the Pathogenesis of Type 2 Diabetes Mellitus.Front Endocrinol (Lausanne). 2018 Nov 26;9:704. doi: 10.3389/fendo.2018.00704. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30542324 Free PMC article. Review.
-
Current Drug Repurposing Strategies for Rare Neurodegenerative Disorders.Front Pharmacol. 2021 Dec 21;12:768023. doi: 10.3389/fphar.2021.768023. eCollection 2021. Front Pharmacol. 2021. PMID: 34992533 Free PMC article. Review.
-
Dysregulation in the Unfolded Protein Response in the FGR Rat Pancreas.Int J Endocrinol. 2020 Jan 20;2020:5759182. doi: 10.1155/2020/5759182. eCollection 2020. Int J Endocrinol. 2020. PMID: 32411226 Free PMC article.
-
Activating transcription factor 6 protects insulin receptor from ER stress-stimulated desensitization via p42/44 ERK pathway.Acta Pharmacol Sin. 2011 Sep;32(9):1138-47. doi: 10.1038/aps.2011.75. Epub 2011 Aug 15. Acta Pharmacol Sin. 2011. PMID: 21841811 Free PMC article.
References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. - PubMed
-
- Urano F, Bertolotti A, Ron D. IRE1 and efferent signaling from the endoplasmic reticulum. J Cell Sci. 2000;113:3697–3702. - PubMed
-
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. - PubMed
-
- Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

